Fission and Fusion
advertisement

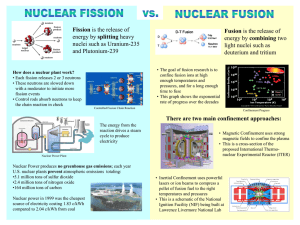
Nuclear Reactions Fission and Fusion CS 4.4 State that in fission a nucleus of large mass splits into 2 nuclei of smaller mass numbers, usually with the release of neutrons. CS 4.5 State that fission may be spontaneous or induced by neutron bombardment. CS 4.6 State that in fusion, 2 nuclei combine to form a nucleus of larger mass number. CS 4.7 Explain, using E = mc2, how the products of fission and fusion acquire large amounts of kinetic energy. CS 4.8 Carry out calculations using E = mc2 for fission and fusion reactions. Fission When atoms are bombarded with neutrons, their nuclei splits into 2 parts which are roughly equal in size. Nuclear fission in the process whereby a nucleus, with a high mass number, splits into 2 nuclei which have roughly equal smaller mass numbers. During nuclear fission, neutrons are released. Nuclear Fission There are 2 types of fission that exist: 1. Spontaneous Fission 2. Induced Fission Spontaneous Fission Some radioisotopes contain nuclei which are highly unstable and decay spontaneously by splitting into 2 smaller nuclei. Such spontaneous decays are accompanied by the release of neutrons. Induced Fission Nuclear fission can be induced by bombarding atoms with neutrons. The nuclei of the atoms then split into 2 equal parts. Induced fission decays are also accompanied by the release of neutrons. The Fission Process A neutron travels at high speed towards a uranium-235 nucleus. 1 0 n 235 92 U The Fission Process A neutron travels at high speed towards a uranium-235 nucleus. 1 0 n 235 92 U The Fission Process A neutron travels at high speed towards a uranium-235 nucleus. 1 0 n 235 92 U The Fission Process The neutron strikes the nucleus which then captures the neutron. 1 0 n 235 92 U The Fission Process The nucleus changes from being uranium-235 to uranium-236 as it has captured a neutron. 236 92 U The Fission Process The uranium-236 nucleus formed is very unstable. It transforms into an elongated shape for a short time. The Fission Process The uranium-236 nucleus formed is very unstable. It transforms into an elongated shape for a short time. The Fission Process The uranium-236 nucleus formed is very unstable. It transforms into an elongated shape for a short time. The Fission Process It then splits into 2 fission fragments and releases neutrons. 1 0 n 1 0 n 1 0 n 141 56 Ba 92 36 Kr The Fission Process It then splits into 2 fission fragments and releases neutrons. 1 0 n 1 0 n 1 0 n 141 56 Ba 92 36 Kr The Fission Process It then splits into 2 fission fragments and releases neutrons. 1 0 n 1 0 n 1 0 n 141 56 Ba 92 36 Kr The Fission Process It then splits into 2 fission fragments and releases 1 neutrons. 0n 141 56 Ba 1 0 92 36 Kr 1 n Nuclear Fission Examples 235 1 141 92 1 235 1 138 96 1 U n + 92 0 U n + 92 0 Ba Kr n 3 + + 56 36 0 Cs Rb n 2 + + 55 37 0 Energy from Fission Both the fission fragments and neutrons travel at high speed. The kinetic energy of the products of fission are far greater than that of the bombarding neutron and target atom. EK before fission << EK after fission Energy is being released as a result of the fission reaction. Energy from Fission 235 1 U n + 92 0 138 96 Cs Rb n 2 + + 55 37 0 Element Atomic Mass (kg) 235 U 92 3.9014 x 10-25 138 Cs 55 2.2895 x 10-25 96 Rb 37 1.5925 x 10-25 1 0n 1 1.6750 x 10-27 Energy from Fission Calculate the total mass before and after fission takes place. The total mass before fission (LHS of the equation): 3.9014 x 10-25 + 1.6750 x 10-27 = 3.91815 x 10-25 kg The total mass after fission (RHS of the equation): 2.2895 x 10-25 + 1.5925 x 10-25 + (2 x 1.6750 x 10-27) = 3.9155 x 10-25 kg Energy from Fission The total mass before fission = 3.91815 x 10-25 kg The total mass after fission = 3.91550 x 10-25 kg total mass before fission > total mass after fission Energy from Fission mass difference, m = total mass before fission – total mass after fission m = 3.91815 x 10-25 – 3.91550 x 10-25 m = 2.65 x 10-28 kg This reduction in mass results in the release of energy. Energy Released The energy released can be calculated using the equation: E = mc2 Where: E m c2 E = energy released (J) m = mass difference (kg) c = speed of light in a vacuum (3 x 108 ms-1) Energy from Fission Calculate the energy released from the following fission reaction: 235 1 U n + 92 0 m = 2.65 x 10-28 kg c = 3 x 108 ms-1 E=E 138 96 1 Cs Rb n 2 + + 55 37 0 E = mc2 E = 2.65 x 10-28 x (3 x 108)2 E = 2.385 x 10-11 J Energy from Fission The energy released from this fission reaction does not seem a lot. This is because it is produced from the fission of a single nucleus. Large amounts of energy are released when a large number of nuclei undergo fission reactions. Energy from Fission Each uranium-235 atom has a mass of 3.9014 x 10-25 kg. The total number of atoms in 1 kg of uranium-235 can be found as follows: No. of atoms in 1 kg of uranium-235 = 1/3.9014 x 10-25 No. of atoms in 1 kg of uranium-235 = 2.56 x 1024 atoms Energy from Fission If one uranium-235 atom undergoes a fission reaction and releases 2.385 x 10-11 J of energy, then the amount of energy released by 1 kg of uranium-235 can be calculated as follows: total energy = energy per fission x number of atoms total energy = 2.385 x 10-11 x 2.56 x 1024 total energy = 6.1056 x 1013 J Nuclear Fusion In nuclear fusion, two nuclei with low mass numbers combine to produce a single nucleus with a higher mass number. 2 3 H H + 1 1 4 1 Energy He n + + 2 0 The Fusion Process 2 1H 3 1H The Fusion Process 2 1H 3 1H The Fusion Process 2 1H 3 1H The Fusion Process 2 1H 3 1H The Fusion Process The Fusion Process The Fusion Process The Fusion Process The Fusion Process 1 0 4 2 He n The Fusion Process 1 0 4 2 He n The Fusion Process 1 0 4 2 He n The Fusion Process 1 0 4 2 He n Energy from Fusion 2 3 H H + 1 1 Element 4 1 Energy He n + + 2 0 Atomic Mass (kg) 2 3.345 x 10-27 3 1H 5.008 x 10-27 4 He 2 6.647 x 10-27 1H 1 0n 1.6750 x 10-27 Energy from Fusion Calculate the following: • The mass difference. • The energy released per fusion. Energy from Fusion 2 3 H H + 1 1 4 1 Energy He n + + 2 0 The total mass before fusion (LHS of the equation): 3.345 x 10-27 + 5.008 x 10-27 = 8.353 x 10-27 kg The total mass after fission (RHS of the equation): 6.647 x 10-27 + 1.675 x 10-27 = 8.322 x 10-27 kg Energy from Fusion m = total mass before fission – total mass after fission m = 8.353 x 10-27 – 8.322 x 10-27 m = 3.1 x 10-29 kg Energy from Fusion 2 3 H H + 1 1 m = 3.1 x 10-29 kg c = 3 x 108 ms-1 E=E 4 1 Energy He n + + 2 0 E = mc2 E = 3.1 x 10-29 x (3 x 108)2 E = 2.79 x 10-12 J The energy released per fusion is 2.79 x 10-12 J.