DNA structure
advertisement

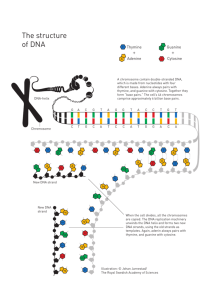
DNA – An Introduction DNA Deoxyribonucleic acid In the middle of the 1900’s biologists were wondering how genes work, what they are made of and how they determine the characteristics of organisms. If the structures that carry genetic information could be identified, it might be possible to understand how genes control the inherited characteristics of living things. DNA and the genetic code are the basis of the structure and function of all living things on the planet. A great deal of attention will be paid to the DNA molecule in the 21st century. The better acquainted you are with the molecule, including what it is, where it is found and what it does, the better you will understand the rapid changes as they take place in the world around you. The Human Genome Project is just such an example. It is the mammoth undertaking of finding the precise location and function of all 20,000 to 25,000 or so genes that exist with in human DNA. With completion has come major breakthroughs in medicine and progress towards gene therapies for people suffering from genetic diseases. DNA DNA stands for deoxyribose nucleic acid This chemical substance is present in the nucleus of all cells in all living organisms DNA controls all the chemical changes which take place in cells The kind of cell which is formed, (muscle, blood, nerve etc) is controlled by DNA The kind of organism which is produced (buttercup, giraffe, herring, human etc) is controlled by DNA 2 WHAT IS A GENE? 2005 2003 DNA Double Helix, Watson & Crick Nature, 1953 Human genome Project The physical and functional unit of heredity that carries information from one generation to the next Chromosomes and DNA Our genes are on our chromosomes. Chromosomes are made up of a chemical called DNA. 7 GENE • Gene were first detected and analyzed by Mendel and subsequently by many other scientist (Mendel stated that physical traits are inherited as “particles”) Mendel did not know that the “particles” were actually Chromosomes & DNA Experiments provided a strong early evidence that genes are usually located on chromosomes. Hank Strikes Again! DNA DNA is often called the blueprint of life. In simple terms, DNA contains the instructions for making proteins within the cell. 9 Proteins.. A quick side journey In living things, the vital components are made up of chiefly proteins, carbohydrates, lipids and nucleic acids. Protein Types: About eight different categories of proteins are found in living things. 1. Transport Proteins – pass ions and vital molecules such as glucose and amino acids across cell membranes. 2. Enzyme Proteins – biological catalysts 3. Antibody Proteins – immunoglobulin antibodies fight bacterial and viral infections. 4. Contractile Proteins – change shape rapidly. Actin and myosin are proteins in muscle tissue that allow animals to move their limbs. 5. Hormone Proteins – regulate the function of various organs. Insulin secreted by the pancreas regulates blood sugar concentrations. 6. Storage Proteins – amino acid depositories. Casein in milk is an example. 7. Receptor Proteins – cell surface proteins that bind with signalling molecules carried in the bloodstream. Adrenalin binds to receptors on the surface of the liver or muscle cells to trigger the release of glucose. 8. Structural Proteins – make up skin, fingernails, hair, ligaments. Proteins are made up of amino acids. There are 20 different amino acids that go into the construction of all proteins. Alanine Side chain Proline ( 1 amino acid wi and not NH2 Side Chain The amino acids link up to one another (like links in a chain) by a peptide bond. Many amino acids linked together is called a polypeptide. Chromosomes are composed of two types of large organic molecules (macromolecules) called proteins and nucleic acids. The NA (nucleic acids) are of two types: DNA and RNA For many years there was considerable disagreement among scientists as to which of these macromolecules carries genetic information. During the 1940s and early 1950s, several elegant experiments were carried out that clearly shows that NA is genetic material rather than protein. More specifically these expt. shows that DNA is genetic material for all living organism except for RNA viruses. The Historical Perspective 1869: Friedrich Miescher Miescher discovered DNA while analyzing pus from discarded bandages. He named the substance “nuclein” but did not realize he was looking at the origin of evolution. Johann Friedrich Miescher The Historical Perspective 1908: Thomas Hunt Morgan Morgan worked with Drosophila and found that genes were located on chromosomes. However, Morgan did not know whether it was the DNA or the histone proteins that are the actual genes. T.H. Morgan The Historical Perspective 1947: Erwin Chargaff Discovered what is now known as Chargaff’s Rules. In DNA: % of Adenine ≈ % of Thymine % of Cytosine ≈ % of Guanine Relative Proportions (%) of Bases in DNA Organism A T G C Human 30.9 29.4 19.9 19.8 Chicken 28.8 29.2 20.5 21.5 Grasshopper 29.3 29.3 20.5 20.7 Sea Urchin 32.8 32.1 17.7 17.3 Wheat 27.3 27.1 22.7 22.8 Yeast 31.3 32.9 18.7 17.1 E. coli 24.7 23.6 26.0 25.7 Erwin Chargaff Chargaff’s Rule A=T and G= C Chargraff’s Rule: • • Adenine and Thymine always join together A T C G Cytosine and Guanine always join together 20 The Historical Perspective 1953: Watson and Crick (and Franklin and Wilkins) Rosalind Franklin and Maurice Wilkins Maurice Wilkins Rosalind Franklin Francis Crick James Watson used X-ray crystallography to learn about the structure of DNA. The image to the right shows a uniform Xshape, suggesting a helix shape with a consistent width. So where do Watson and Crick come in? The Historical Perspective Watson and Crick used the work of Franklin and Wilkins to create models of DNA, eventually figuring out its structure. They still deserve credit, but Wilkins and Franklin deserve just as much. Unsuccessful until 1953, Watson was shown a copy of Franklin’s X-ray pattern. “The instant I saw the picture my mouth fell open and my pulse began to race.”James Watson Within weeks Watson and Crick had figured out the structure of DNA Published their results in a historic one page paper in April of 1953. Watson and Crick later discovered what held the two strands together. Hydrogen bonds could form between certain nitrogen bases and provide enough force to hold the two strands together. Hydrogen bonds could only form between certain base pairs adenine and thymine and guanine and cytosine. This principal is called Base pairing. In 1962 James Watson (b. 1928), Francis Crick (1916–2004), and Maurice Wilkins (1916–2004) jointly received the Nobel Prize in physiology or medicine for their 1953 determination of the structure of deoxyribonucleic acid (DNA). Because the Nobel Prize can be awarded only to the living, Wilkins’s colleague Rosalind Franklin (1920–1958), who died of cancer at the age of 37, could not be honored. Of the four DNA researchers, only Rosalind Franklin had any degrees in chemistry. 26 So what comes out of all that work? The classic DNA structure: a double helix. Meaning it looks like that spiral staircase in Gattaca. Coincidence? I think not. DNA STRUCTURE Nucleic acids first called “nuclein” because they were isolated from cell nuclei by Miescher in 1869 Each nucleotide is composed of (1) a Phosphate group (2) a five – carbon sugar (or Pentose), and (3) a nitrogen containing compound called a base. Nucleotides These are the units that make up a DNA molecule. Made of three parts: 5 carbon sugar (deoxyribose) phosphate group nitrogen bases The Shape of the Molecule DNA is a very long polymer. The basic shape is like a twisted ladder or zipper. This is called a double helix. 30 One Strand of DNA phosphate The backbone of the molecule is alternating phosphates and deoxyribose sugar deoxyribose The teeth are nitrogenous bases. bases 31 DNA Structure Review Surrounding the base pairs and forming the sides of the “ladder” is a sugar-phosphate backbone. The backbone is made of a sugar (deoxyribose) and a phosphate group, alternating and in reverse order from the other strand. Backbone is linked by phosphodiester bonds. The end of DNA with the phosphate on top is the 5’ (“five prime”) end. The other end of the backbone is the 3’ (“three prime”) end. 3’ and 5’? Huh? 3’ and 5’ get their names from the pentose sugar’s carbon atoms. Each carbon in pentose is numbered and has a specific job in the formation of DNA. Carbon 1 = base attachment Carbon 2 = oxygen (ribose) or not (deoxyribose)? Carbon 3 = another nucleotide attachment Carbon 4 = completes ring Carbon 5 = phosphate attachment This is important. http://users.rcn.com/jkimball.ma.ultranet/BiologyPages/P/Pentose. http://www.synapses.co.uk/genetics/pentose1.gif One more time, because “important.” Oxygen, not a zero. In DNA, the sugar is 2-deoxyribose (thus the name deoxyribonucleic acid) In RNA, the sugar is ribose (thus ribonucleic acid). Ribose has a hydroxyl group (OH) on carbon 2, while deoxyribose is without an oxygen on carbon 2 (H). One Strand of DNA nucleotide One strand of DNA is a polymer of nucleotides. One strand of DNA has many millions of nucleotides. 36 Two Kinds of Bases in DNA Pyrimidines are single ring bases. Purines are double ring bases. N N C O C C N C N N C C C N N C N C 37 Thymine and Cytosine are pyrimidines Thymine and cytosine each have one ring of carbon and nitrogen atoms. N O C C O C C N C thymine N O C C N C N C cytosine 38 Adenine and Guanine are purines Adenine and guanine each have two rings of carbon and nitrogen atoms. N C Adenine N C C N O N C N N C N C C C N Guanine C N N C 39 There are four different bases commonly found in DNA: Adenine Guanine Thymine and Cytosine. RNA also contains adenine, guanine and cytosine, but has different base, uracil in the place of thymine. DNA RNA Deoxyribose is the sugar Double stranded Contains the bases A T Ribose is the sugar Single stranded Contains the bases A U (Uracil replaces Thymine) G G C C 41 4 kinds of nitrogen bases in DNA 1. Adenine (A) 2. Guanine (G) 3. Cytosine (C) 4. Thymine (T) Two Stranded DNA Remember, DNA has two strands that fit together something like a zipper. The teeth are the nitrogenous bases but why do they stick together? 43 N Hydrogen Bonds The bases attract each The bonds between cytosine and guanine are shown here with dotted lines C C N C C C C N N N C O but there are millions and millions of them in a single molecule of DNA. C Hydrogen bonds are weak N N C other because of hydrogen bonds. N O 44 Hydrogen Bonds, cont. When making hydrogen bonds, cytosine always pairs up with guanine Adenine always pairs up with thymine N O C C O C C N C Adenine is bonded to thymine here 45 Chargraff’s Rule: • Adenine and Thymine always join together A • T •Cytosine and Guanine always join together C G 46 14 THE DOUBLE HELIX bases sugar-phosphate chain DNA by the Numbers Each cell has about 2 m of DNA. The average human has 75 trillion cells. The average human has enough DNA to go from the earth to the sun more than 400 times. DNA has a diameter of only 0.000000002 m. The earth is 150 billion m or 93 million miles from the sun. 48 49