Chapter 14
advertisement

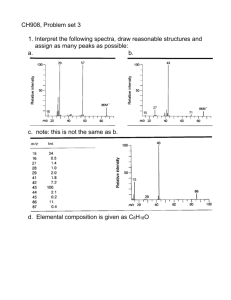
Chapter 14 Mass Spectroscopy Mass Spectrometer Positive ions are detected. Neutral species are undetected. p.545 Parent peak due to molecular radical cation. Figure 14.2, p.546 Detecting nitrogen, N Consider some simple molecules and their nominal mass. CH4 16 CH3NH2 29 CH3OH 32 CH3F 34 CH3Cl 50, 52 CH3SH 48 What is unusual about the N compound? The parent peak should have an odd mass for an odd number of nitrogens. p.546 One way to distinguish between molecules having the same about the same mass is to measure their mass very precisely. About the same mass for these species formed from the most common isotopes. In these cases we can actually determine the molecular formula from high resolution mass spectroscopy. p.547 35.453 = (100 * 34.9689 + 31.98 * 36.9659) / 131.98 Recall that the atomic weight is the average mass for all isotopes found in nature. Table 14.1, p.548 For example chlorine…. Low resolution mass spec does not involve itself with precise mass measurements. Low resolution examines the various peaks produced. First consider the parent radical cation: if an element has two naturally occurring isotopes then two peaks will be produced. Bromine has two isotopes 79Br and 81Br in about equal amounts. Obtain two peaks at 122 and 124. Figure 14.4, p.550 Further comments on presence of chlorine and bromine. Both Cl and Br have two common isotopes separated by two mass units. Given the natural abundances we may calculate the ratio of the M and M+2 peaks for various combinations of Cl and Br being present. The presence of peaks at X, X+2… for the molecular ion or Ratio offragment peaks calculated hopefully as with close to&the expected ratio37isCltaken 35Cl79Br 37Cl79Br 35Cl 81Br 81Br as indication of Cl or Br. 1.00 *1.00 .324 *1.00+1.00 *.979 .324*.979 Ratio of 1.00 peaks calculated as .243 .767 35Cl 35Cl37Cl & 37Cl35Cl 37Cl 2 2 1.00*1.00 1.00*.324+.324*1.00 .324*.324 Molecular Peaks, M+1 Have seen that for Cl and Br, having two common isotopes, two radical cation peaks produced. What about other elements having more than one isotope? We know what the isotopes are and their natural occurrence. For the M+1 peak, one atom must be using an isotope heavier by one. Here is the data. We will use isotopic occurrence data for H, C, O for the M + 1 peak. The M+2 peak Recap: The M+1 peak has contributions from one atom being a heavier isotope by 1. The M+2 peak can have contributions from •One atom being a heavier isotope by 2. •Two atoms being heavier by 1 each. M+2 peak, contributions from one atom and two atoms. Recap: The M+1 peak has contributions from one atom being a heavier isotope by 1. (M+1)/M = ca. 1.1% * no. of C atoms + 0.36% * no. of N atoms The M+2 peak can have contributions from two sources •One atom being a heavier isotope by 2. Mainly O (excluding S, Cl and Br) •Two atoms being heavier by 1 each. Mainly C atoms. (M+2)/M = ca. (0.20% * no. of O atoms) + (1.1 * no. of C atoms)2/200% Example 1: C5H5N [(A + 1)+]/[A+] = 5 x 1.1% + 1 x 0.36% = 5.9% [(A + 2)+]/[A+] = 5.52/200 % = 0.15% Example 2: C7H5O [(A + 1)+]/[A+] = 7 x 1.1% = 7.7% [(A + 2)+]/[A+] = 7.72/200 % + 0.20% = 0.50% Technique to obtain molecular formula using intensities of M, M+1, M+2 peaks. Example. Given the data. Peak Intensity 150 (M) 100 151 (M+1) 10.2 152 (M+2) 0.88 Looking at M+2 there is no Br, Cl or S. There could be oxygen. Even mass for M means there could only be even number of Nitrogen Consider the M+1 peak, nominal mass + 1. If we know the formula we should be able to calculate the relative intensity of that peak due to the contributions from each of the atoms present. Here are the major contributors to M+1. Here are major contributors to M+2. Technique to obtain molecular formula using intensities of M, M+1, M+2 peaks. Example. Given the data. Peak Intensity 150 (M) 100 151 (M+1) 10.2 152 (M+2) 0.88 Equations M+1: (1.11% x # of C) + (0.38 x # of N+ small contributions from O M+2: (0.20 x # of O) + (1.1 x # of C)2/200 Examine reasonable formulae. Calculate M+1, M+2 peaks M+1 M+2 We can have 0 or 2 nitrogens. Even number. C7H10N4 9.25 0.38 We can have 0,1,2,3,4 oxygens. 0.88/0.2 < 5 Can have 0,1,2,3,4,5,6,7,8,9 carbons. 10.2/1.11 <10 C8H10N2O 9.61 0.61 C9H10O2 9.96 0.84 Find molecular formulas having reasonable M+1 peaks C9H14N2 10.71 0.52 Return to Fragmentation of Molecular Radical Cation, M+ The highly energetic radical/cation can undergo fragmentation. First distinguish between some species •Radical/cation produced by ejection of electron from structure. It contains all the atoms of the original molecule just minus one electron. Example C H +. 4 10 •Carbocation which is not a radical, is electron deficient and is a fragment of a stable molecule. Example C4H9+ •Radical is not charged and is a fragment of a stable molecule. Example C4H9. Example. Identify this molecule m/e Abundance 1 <0.1 16 1.0 17 21 18 100 19 0.15 20 0.22 H2O Ejection of an H Molecular radical ion Due to heavier isotopes Example 2 m/e Abundance 12 3.3 13 4.3 14 4.4 15 0.07 16 1.7 Oxygen 28 31 H ejection 29 30 31 32 100 89 1.3 0.21 CH2O carbon parent Heavier isotopes Fragmentation of the radical/cation can lead to 1. A radical and carbocation as separate species. Usually a bond is split. Choice of bond to split is frequently controlled by the carbocation stability. Carbocation, CH3CH2+ Radical/ cation, . C4H10+. Radical, CH3CH2. Remember only the positive species are detected. Neutral species are invisible. 2. Rearrangements can occur including elimination of neutral molecules to produce a different radical/cation. Radical/ cation, H2O + C5H12O+. OH Radical/ cation, C5H10+. H p.550 How to think about it… For this . fragmentation Think of it this way… One electron in this bond. When it splits we get a carbocation (observable in MS) and a radical (not observable in MS). This C becomes + The fragmentation is written this way. This C bears the . In fragmentation to produce a carbocation stability of the carbocation is an important factor in determining where the fragmention will occur. p.551 For simple linear alkanes fragmentation will occur towards the middle of the chain. Figure 14.5, p.552 Caution. Sometimes a peak will occur which cannot be explained such as the ethyl peak below. Figure 14.6, p.552 Technique: recall that the neutral molecules split out do not produce a peak. The mass of the neutral particle (invisible) may sometimes be obtained by subtracting the mass of the newly formed positive ion (detected) from the mass of the original radical carbocation. In this example the parent molecule has mass of 84. The mass of a positive fragment is 56. The peak at 56 is subtracted from the mass of the original ion, 84, yielding 28, the mass of ethylene which is taken as the invisible neutral molecule. Figure 14.7, p.553 Ionization followed by fragmentation . Ejection of electron Splitting out of ethylene. fragmentation Radical/cation p.553 Alkenes can yield allylic stabilized carbocations by fragmentation, splitting out a radical. .+ CH 3 Difference is 15, the methyl radical Alcohols have several characteristic fragmentation patterns. 1. An alcohol radical/cation can undergo a fragmentation to produce a radical and a resonance stabilized carbocation. . + R R R' C O H R' R" . C R" O H R' C O H R" The one “electron bond” p.555 2. Alcohol radical/cations can split out water to produce a new alkene radical/cation which may be detected. Here is an example demonstrating both processes. Elimination of water. Elimination of propyl radical. 74 – 56 = 18 (water). 74 – 31 = 43 (C3H7) Figure 14.10, p.555 Aldehydes and Ketones Several characteristics reactions the radical/cations 1. a cleavage: break bond to carbonyl group Note that an a cleavage of an aldehyde could produce a peak at M – 1 by eliminating H atom. This is useful in distinguishing between aldehydes and ketones. 2. McLafferty Rearrangement: splitting out an alkene (neutral molecule) and producing a new radical/cation. Note that the process involves a six membered ring for a transition state. p.557 Mass Spec of 2-octanone displays both a cleavage and McLafferty The “invisible” radical C6H13 “Invisible” pent-1-ene CO+ CH3 resulting from a cleavage at this bond. CH3 radical CH3CH2CH2CH2CH2CH2CO+ resulting from a cleavage here. Carboxylic Acids can also undergo a cleavage and McLafferty rearrangement. The peak at 60 is usually prominent for a carboxylic acid. Likewise for esters, 2 a cleavages (around C=O) and McLafferty C An unexpected observation. p.559 Figure 14.14, p.559 Some general principles The relative height of the parent radical cation peak is greatest for straight chain compounds and decreases for branched structures. Easier cleavage. Cleavage is favored at branched carbons due to increased stability of substituted carbocations. Double bonds or aromatic rings stabilize the parent radical cation increasing the size of its peak. Double bonds (aromatic rings) favor cleavage yielding allylic (benzylic or tropylium) carbocations. R CH2 H R H Bonds beta to a hetero atom having lone pairs are frequently cleaved. Elimination of small, stable molecules (water, alkenes, etc.) can occur to yield a new radical cation. N H H H Example Given spectra for an unknown compound: IR, MS, and NMR. Identify the compound. First, let’s do the MS Mass Spectrum Now the IR…. Base peak, the largest, others are relative to it. Want M+1 and M+2 relative to M not base peak. Observations: 1. Parent mass is even. Even number of nitrogens: 0, 2, 4 2. No Br or Cl since M+2 is much too small. 3. At most about 9 carbons since 9.9/1.1 =9. What molecular formulas could we have with good values for M+1 between 9 and 11? Molecular peak, parent peak. Best fit for both M+1 and M+2. Maybe aromatic! Infra Red. Assumed formula C9H10O2 Carbonyl, no OH C-O stretch Looks as if it may be an ester with an aromatic group. Next the NMR…. NMR . Assumed formula C9H10O2 First the hydrogen counts. Chemical Arithmetic. Assumed formula C9H10O2 Consistent with the lack of splitting could be either. C9H10O2 O O - C6H5 - CH2 - CH3 Tentatively identified parts, Subtract to get CO2 O O