16ppt
advertisement
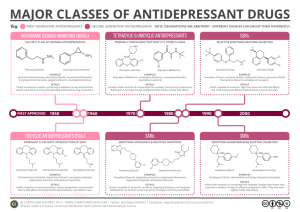
Anxiolytics, Continued • Benzodiazepines • Buspirone (BuSpar®) • Antiepileptics • SSRI, SNRI antidepressants Busprione (BuSpar®) Buspirone (BuSpar®) – Selective serotonin 5-HT1A agonist • Lacks risks of dependence and withdrawal compared to benzodiazepines • Antianxiety effects after several weeks of treatment (contrast to benzodiazepines – fast acting) • Low oral bioavailability (4-5%) due to extensive first pass metabolism by CYP3A4 • May be taken with grapefruit juice to increase bioavailability (furanocoumarins inhibit CYP3A4) Antiepileptics (Anticonvulsants) – Expanded Use to Other Indications • Bipolar disorder (“mood stabilizer”) • Alcohol withdrawal • Chronic pain • Depression • Anxiety disorders Antiepileptics (Anticonvulsants) – Mechanisms of Action Conventional antiepileptics 1. Block Na+ channels, reducing release of glutamate (excitatory NT) or 2. Enhance GABA function Glutamate excitatory NT GABA inhibitory NT Antiepileptics for Anxiety Disorders and Pain Neurontin® and Lyrica® approved for neuropathic and chronic pain, anxiety disorders Generalized anxiety disorder (GAD), social anxiety disoder (SAD), panic disorder Gabapentin (Neurontin®) Increases GABA synthesis via enzyme modulation Does not bind to GABA receptor Pregabalin (Lyrica®) More potent than gabapentin Decreases release of glutamate, norepinephrine, substance P Does not affect GABA neurotransmission Antidepressants First marketed in late 1950s Estimated 10% of Americans are using an antidepressant Higher use among women, and non-Hispanic white people Antidepressants also indicated for many anxiety disorders. Stress believed to be most significant cause of depression. http://www.health.harvard.edu/blog/astounding-increase-in-antidepressant-use-by-americans-201110203624 Efficacy of Antidepressants Greater in Severe or Chronic Cases HDRS - Hamilton Depression Rating Scale - method of measuring severity of depression. 17 item questionnaire – maximum score of 52 (higher score = greater severity) Antidepressants – Theories on Mechanism of Action Classically, depression was attributed to a deficiency in the neurotransmitters serotonin, norepinephrine and dopamine (“monoamines”). Prolonging the presence of NT in synaptic cleft was thought to be responsible for improved mood. Weakness of “monoamine” model: 1. NT changes occur rapidly though clinical effects require weeks of treatment. 2. >40% of patients do not respond to monoaminergic antidepressants. 3. Single IV infusion of ketamine (glutamate NMDA antagonist) can rapidly relieve symptoms. Antidepressants – Theories on Mechanism of Action Neurogenic theory of depression Current view of antidepressant action is based neuronal repair and increased neurogenesis. 1. The brain is capable of synthesizing new neurons (hippocampus, frontal cortex) 2. Existing neurons are able to repair or remodel themselves Support for theory Stress reduces hippocampal and frontal cortical neurogenesis and damages existing neurons. Antidepressants can reverse hippocampal shrinkage. Molecular Mechanism of Action of Long-Term Antidepressant Treatment Second-messenger systems activate downstream production of proteins controlling gene expression. BDNF = Brain Derived Neurotropic Factor. Protein supporting survival and grown of neurons and synapses Therapeutic Delay in Clinical Effect of Antidepressants Due to Time Required for New Neurons to Develop, Mature, and Become Functional • BNDF levels decreased in depressed patients • Antidepressants, exercise, light therapy, electroconvulsive therapy can increase BDNF http://www.nature.com/mp/journal/v12/n12/fig_tab/4002075f1.html#figure-title Antidepressant Drug Classes Monoamine oxidase inhibitors (MAOIs) Tricyclic antidepressants (TCAs) Selective serotonin reuptake inhibitors (SSRIs) Serotonin-norepinephrine reuptake inhibitors (SNRIs) Others Monoamine Oxidase Inhibitors (MAOIs) – First Generation Antidepressants MAOIs increase the levels of released serotonin, norepinephrine and dopamine by blocking the enzyme that breaks them down (via oxidation) in the presynaptic neurons. norepinephrine norepinephrine aldehyde ammonia Monoamine Oxidase (MAO) – Two Subtypes MAO-A: Metabolizes serotonin, norepinephrine, dopamine, tyramine MAO-B: Metabolizes dopamine and phenethylamine MAOIs - a partial list Isocarboxazid (Marplan®) - Non-selective MAO-A/MAO-B inhibitor Moclobemide (Aurorix®, Manerix®) – Selective MAO-A inhibitor Selegiline (Deprenyl®, Eldepryl®, Emsam®) – Selective MAO-B inhibitor Monoamine Oxidase Inhibitors (MAOIs) • MAOIs most efficacious drugs developed for depression (developed late 1950s) • Potentially serious side effects and drug-drug interactions limit use (last line of treatment) • Older MAOIs – irreversible inhibitors , covalently modified enzymes (MAO-A, MAO-B). Enzyme activity resumes after ca. 2 weeks, when new enzyme synthesized. • Newer MAOIs are reversible inhibitors, some are selective for one subtype. MAO-B selective reversible inhibitors do not require the same dietary restrictions. Monoamine Oxidase Inhibitors (MAOIs) – Dietary Restrictions Most MAOIs require avoiding tyramine-containing foods, since tyramine is oxidized by MAO. Inhibition of MAO can result in high levels of tyramine, leading to hypertensive crisis (high blood pressure, often termed the “cheese effect”) tyramine Monoamine Oxidase Inhibitors (MAOIs) – Drug-Drug Interactions Lethal interactions can occur with MAOIs and some psychoactive drugs that affect serotonin levels, such as: • SSRIs • MDMA • Tricyclics Dose reductions required for some drugs, such as those that affect epinephrine, norepinephrine, or dopamine levels. Tricyclic Antidepressants (TCAs) – Another Class of First-Generation Antidepressants • Tricyclic antidepressants were developed in the late 1950s • Categorized by their chemical structure • Most act as serotonin and norepinephrine reuptake inhibitors. Imipramine (Tofranil®) Desipramine (Norpramin®) Nortriptyline (Pamelor®) Tricyclic Antidepressants Largely Replaced by SSRIs and SNRIs Due to Side Effects • TCAs also block postsynaptic receptors for histamine and acetylcholine • Histamine receptor blockade results in drowsiness and sedation (similar to diphenhydramine) • Acetylcholine receptor blockade leads to confusion, memory and cognitive impairments, dry mouth, blurred vision, increased heart rate, urinary retention. • Nortriptyline and desipramine generally favored over other TCAs due to less sedation and fewer anticholinergic side effects • Overdose (e.g. suicide attempts) can lead to cardiotoxicity Selective Serotonin Reuptake Inhibitors (SSRIs) Fluoxetine (Prozac®) Paroxetine (Paxil®) Sertraline (Zoloft®) Fluvoxamine (Luvox®) Citalopram (Celexa®) Escitalopram (Lexapro®) Vilazodone (Viibryd®) Clinical differences between the SSRIs is small, though pharmacokinetics and receptor selectivities differ. SSRIs - similar efficacy as older antidepressants. Fewer side effects with SSRIs versus older ADMs. SSRI efficacy of ca. 17% versus placebo. Selective Serotonin Reuptake Inhibitors (SSRIs) SSRIs block the presynaptic transporter for serotonin reuptake, resulting in higher levels of serotonin in the synaptic cleft. SSRIs do not block postsynaptic serotonin receptors. SSRIs Therapeutic Actions and Side Effects Due to Actions of Serotonin at Post-Synaptic Receptors 5-HT1A Antidepressant and anxiolytic effects 5-HT2 Insomnia, anxiety, agitation, sexual dysfunction, serotonin syndrome at higher doses or when combined with another serotonergic agent. Same receptor LSD and other psychedelics target. 5-HT3 Nausea SSRI Discontinuation Syndrome SSRIs not abuse-prone, though withdrawal symptoms can occur upon abrupt termination. Onset within 2-5 days; symptoms persist 2-4 weeks. Symptoms often associated with discontinuation of paroxetine (Paxil®). Fluoxetine (Prozac®) has a long half-life, reducing discontinuation syndrome symptoms. Flulike symptoms, insomnia, nausea, imbalance, sensory disturbances, hyperarousal Serotonin-Norepinephrine Reuptake Inhibitors (SNRIs) Development – Was believed that actions at two different synaptic sites may improve or maintain efficacy while limiting side effects Venlafaxine (Effexor®) Duloxetine (Cymbalta®) ----------------------------------------Venlafaxine – significant sexual side effects, can increased blood pressure, can trigger manic state in patients with bipolar disorder Duloxetine – approved for neuropathic pain, may induce manic episode in bipolar disorder Mirtazepine (Remeron®) – Alternate Mechanisms of Action • Blocks autoreceptors (feedback mechanism) causing increase in release of norepinephrine and serotonin • Blocks 5-HT2 and 5-HT3 receptors – hence avoiding several side effects of SSRIs (anxiety, insomnia, agitation, nausea, sexual dysfunction) --------------------------------Side effects include drowsiness (often limiting), increased appetite and weight gain Trazodone (Desyrel®, Oleptro®) – An Atypical Antidepressant Partial agonist at 5HT1A, antagonist at other 5HT receptors Most common side effects – drowsiness, decreased blood pressure, priaprism One of the most prescribed prescriptions for insomnia. Doses lower than those used for antidepressant activity for sleep (t1/2 = 7 h). Buproprion (Wellbutrin®) – Dopamine and Norepinephrine Reuptake Inhibitor • Monotherapy or add-on therapy for depression • Clinical effects similar to psychostimulants with low abuse potential • Lacks side effects associated with SSRIs (sexual side effects, weight gain) • Side effects can include anxiety, restlessness, tremor, insomnia, seizures • Also marketed as a smoking cessation (Zyban®)