Energy - Cloudfront.net
advertisement
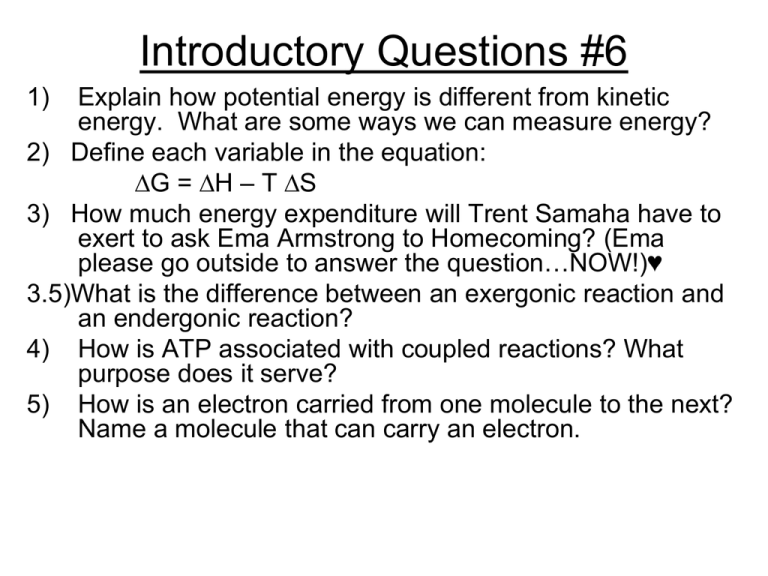
Introductory Questions #6 1) Explain how potential energy is different from kinetic energy. What are some ways we can measure energy? 2) Define each variable in the equation: ∆G = ∆H – T ∆S 3) How much energy expenditure will Trent Samaha have to exert to ask Ema Armstrong to Homecoming? (Ema please go outside to answer the question…NOW!)♥ 3.5)What is the difference between an exergonic reaction and an endergonic reaction? 4) How is ATP associated with coupled reactions? What purpose does it serve? 5) How is an electron carried from one molecule to the next? Name a molecule that can carry an electron. Chapter 8 An Introduction to Energy & Metabolism (Pages 141-159) Topics: •Thermodynamic Laws •Catabolism & Anabolism (metabolism) •Exergonic vs. Endergonic Reactions •Free Energy •ATP Cycle & Energy Coupling •Enzyme (structure & function) Main Topics to Cover-Ch. 8 • • • • • • • • Potential vs. Kinetic Energy First Two Laws of Thermodynamics Entropy, Enthalpy, and Free Energy Endergonic vs. Exergonic Reactions Anabolism & Catabolism = Metabolism Energy Coupling: Oxidation/Reduction ATP; Structure & Function Enzymes Structure & Function (Lab #6) -allosteric, feedback mechanisms, inhibitory sit. Energy is the capacity to perform work • Energy is defined as the capacity to do work • All organisms require energy to stay alive • Energy makes change possible ENERGY AND THE CELL • Living cells are compartmentalized by membranes • Membranes are sites where chemical reactions can occur in an orderly manner • Living cells process energy by means of enzyme-controlled chemical reactions • Kinetic energy is energy that is actually doing work Figure 5.1A • Potential energy is stored energy Figure 5.1B Thermodynamics • Energy (E)~ capacity to do work; Kinetic energy~ energy of motion; Potential energy~ stored energy • Thermodynamics~ study of E transformations • 1st Law: conservation of energy; E transferred/transformed, not created/destroyed • 2nd Law: transformations increase entropy (disorder, randomness) • Combo: quantity of E is constant, quality is not Two laws govern energy conversion • First law of thermodynamics • Energy can be changed from one form to another – However, energy cannot be created or destroyed Figure 5.2A • Second law of thermodynamics • Energy changes are not 100% efficient – Energy conversions increase disorder, or entropy – Some energy is always lost as heat Figure 5.2B Metabolism/Bioenergetics • Metabolism: The totality of an organism’s chemical processes; managing the material and energy resources of the cell • Catabolic pathways: degradative process such as cellular respiration; releases energy • Anabolic pathways: building process such as protein synthesis; photosynthesis; consumes energy Equation Used to Determine Free Energy of a System G = H - T S G: Quantity of Free Energy H: Enthalpy = System’s Total Energy (chemical Bond energy) T: Temperature (absolute temp. in Kelvin units) S: Entropy = Disorder of the system Spontaneous Reaction = G will be negative (energy is released ie. Exergonic.) Non spontaneous Reaction (Endergonic) G will be positive. What happens when G is ZERO ??? Free Energy • Free energy: portion of system’s E that can perform work (at a constant T) • Exergonic reaction: net release of free E to surroundings • Endergonic reaction: absorbs free E from surroundings ATP & Energy Coupling See Pgs. ATP shuttles chemical energy within the cell • ATP is thought as the energy “currency” of a cell • In cellular respiration, some energy is stored in ATP molecules • ATP powers nearly all forms of cellular work • ATP molecules are the key to energy coupling General Facts about ATP • Human use about 99 lbs of ATP each day @rest • Every second 10 million ATP’s are made from ADP • Bacteria has about a one-second supply of ATP Energy Coupling & ATP • Energy coupling: use of exergonic process to drive an endergonic one (ATP) • Adenosine triphosphate (nucleotide w/3 PO4’s) • ATP tail: high negative charge • ATP hydrolysis: release of free Energy • Phosphorylation: binding of the released phosphate to another molecule Introductory Video for Chapter 8 “Metabolism” 1) Name the American Olympiad profiled in this video. What condition did she have? 2) What are the two major reasons why cells use energy? 3) What unique metabolic process does Dr. Margo Haygood discuss in the video? Name the enzyme used for this process. 4) Which two laws of thermodynamics are summarized by Dr. Saltman & Dr. Haygood? 5) Briefly explain how enzymes are able to speed up reactions based on the information from the video. Describe the mechanisms that regulate enzyme activity. The Hydrolysis of ATP • When the bond joining a phosphate group to the rest of an ATP molecule is broken by hydrolysis, the reaction supplies energy for cellular work. G = -32 KJ/mol (-7.6 Kcal/mol) Adenine Phosphate groups Hydrolysis Energy Ribose Adenosine triphosphate Adenosine diphosphate (ADP) Figure 5.4A Figure 5.4C Hydrolysis Energy from exergonic reactions Dehydration synthesis • The ATP cycle Energy for endergonic reactions Example of Energy Coupling w/ATP • Forming the Disaccharide Sucrose involves: glucose + fructose → sucrose (G = +27 KJ/mol) ENDERGONIC Reaction Couple w/hydrolysis of ATP (G = - 32KJ/mol) Occurs in a couple of reaction steps: Reaction#1: Glucose + ATP → glucose-P + ADP **glucose has been phosphorylated **ATP has been hydolyzed Reaction #2: Glucose-P + fructose → Sucrose + Pi (Pi is a low energy inorganic phosphate) • How ATP powers cellular work Reactants Potential energy of molecules Products Work Protein Figure 5.4B Three Functions of ATP Enzymes: Structure & Function See Pgs. 150-157 Lab #3- Enzyme Catalysis w/Catalase Three Parts to the lab: • Establish Baseline Amount of H2O2 • Uncatalyzed Decomposition of H2O2 • Time Trials w/Catalase to determine Rxn rate • Procedure: – – – – – – – 10 ml H2O2 in a beaker 1.0 ml (H2O or Catalase) 10 ml 1 M H2SO4 Mix well Take a 5 ml sample and titrate in KMnO4 Read Initial and final measurements on buret Record Data Enzymes speed up the cell’s chemical reactions by lowering energy barriers • For a chemical reaction to begin, reactants must absorb some energy – This energy is called the energy of activation (EA) – This represents the energy barrier that prevents molecules from breaking down spontaneously Enzymes • Catalytic proteins: change the rate of reactions w/o being consumed • Free E of activation (activation E): the E required to break bonds • Substrate: enzyme reactant • Active site: pocket or groove on enzyme that binds to substrate • Induced fit model • A protein catalyst called an enzyme can decrease the energy barrier Enzyme EA barrier Reactants 1 Figure 5.5A Products 2 A Specific Enzyme Catalyzes each Cellular Reaction • Enzymes are selective – This selectivity determines which chemical reactions occur in a cell How an Enzyme Works-Sucrase How Enzymes Work • http://www.ekcsk12.org/science/aplabrevie w/lab02.htm **Description of Enzyme Lab • Lab Simulation: http://bioweb.wku.edu/courses/Biol114/enzy me/enzyme1.asp Effects on Enzyme Activity • Temperature • pH • Cofactors: inorganic, nonprotein helpers; ex.: zinc, iron, copper • Coenzymes: organic helpers ex. vitamins Enzyme Inhibitors • Irreversible (covalent); reversible (weak bonds) • Competitive: competes for active site (reversible); mimics the substrate • Noncompetitive: bind to another part of enzyme (allosteric site) altering its conformation (shape); poisons, antibiotics Enzyme inhibitors block enzyme action • Inhibitors interfere with enzymes – A competitive inhibitor takes the place of a substrate in the active site – A noncompetitive inhibitor alters an enzyme’s function by changing its shape Substrate Active site Enzyme NORMAL BINDING OF SUBSTRATE Competitive inhibitor Noncompetitive inhibitor ENZYME INHIBITION Figure 5.8 Competitive & Noncompetitive Inhibitors The Cellular environment affects enzyme activity • Enzyme activity is influenced by – temperature – salt concentration – pH • Some enzymes require non-protein cofactors such as: Fe, Zn, Cu, etc. Some Pesticides and Antibiotics inhibit Enzymes • Certain pesticides are toxic to insects because they inhibit key enzymes in the nervous system • Many antibiotics inhibit enzymes that are essential to the survival of disease-causing bacteria – Penicillin inhibits an enzyme that bacteria use in making cell walls Allosteric Enzymes-How they Work Feedback Inhibition Introductory Questions #6 1) Explain how potential energy is different from kinetic energy. What are some ways we can measure energy? 2) Define each variable in the equation: ∆G = ∆H – T ∆S 3) What is the difference between an exergonic reaction and an endergonic reaction? 4) How is ATP associated with coupled reactions? What purpose does it serve? 5) How is an electron carried from one molecule to the next? Name a molecule that can carry an electron. Introductory Questions #7 1) Name three ways that enzyme activity can be measured as mentioned in your lab guidesheet. 2) Explain how the reactivity of pepsin is different from trypsin. (see pg. 154) 3) Give an two examples of a cofactor and two examples of a conenzyme. How are they different? 4) How is a competitive inhibitor different from a non-competitive inhibitor? 5) What is an allosteric enzyme? Energy Transfer in Redox Reactions See Pgs. 161-162 Redox-Defining Terms • Oxidation = Electrons are lost • Reduction = Electrons are gained • These two reactions are complementary and occur simultaneously (they must go together) • Electrons cannot exist in a free state, they must be associated with a molecule • This process allows electrons to be transferred from one molecule to another • Often occur in a series • Essential for cellular respiration & Photosynthesis Electrons are transferred by a carrier molecule by the hydrogen atom • Common electron carrier: NAD+ • NAD+ is Nicotinamide adenine dinucleotide • When NAD+ is reduced it gains an electron and becomes reduced. • The electron losses some of its free energy during the transfer • Expressed as: X-H2 + NAD+ X+ NADH + H+ (oxidized) (reduced) Other Electron Carriers (hydrogen acceptors) • NADP+ (see in photosynthesis) • FAD + (see in the Kreb cycle for cellular respiration) • Cytochromes – embedded in membranes **Remember that: Reduced state more free energy vs. Oxidized state which has less free energy Cool “Fires” Attract Mates and Meals • Fireflies use light, instead of chemical signals, to send signals to potential mates • Females can also use light flashes to attract males of other firefly species — as meals, not mates • The light comes from a set of chemical reactions, the luciferinluciferase system • Fireflies make light energy from chemical energy • Life is dependent on energy conversions





