Nutrition Expedition
advertisement

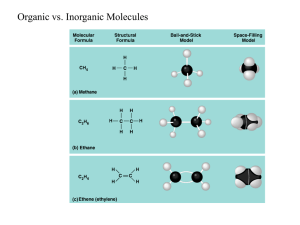
Investigating the three of the four major biological molecules, including structure and function within biological systems. Biological macromolecules are defined as large molecules made up of smaller organic molecules. There are four classes of macromolecules: , , and . Together, these elements make up almost all living things. •Carbohydrates are an essential structural component of living cells and source of energy for animals; includes simple sugars with small molecules as well as macromolecular substances. •They are sugars, starches, and cellulose, which contain CHO and which function primarily in energy storage, energy transport, and plant structure. •A carbohydrate is an organic compound consisting only of carbon, hydrogen and oxygen. Carbohydrates can be viewed as hydrates of carbon, hence their name. •Lipids are an essential structural component in living cells in that they: ohelp with cell membrane structure oconstitute a barrier for the cell ocontrol membrane fluidity ocontrol the flow of material that go in and out of the cell oact as an energy storage (fats stored in adipose tissue) otransmit information in cells ocan act as a Lipid Vitamin required for metabolism. •Fats, oils and waxes are all examples of Lipids, which are molecules necessary for the survival of all living organisms. •A lipid is composed of a glycerol, a type of alcohol, attached to three fatty acid chains composed of a carbon skeleton with attached hydrogen atoms. •Proteins play an important role in the lifespan and quality of human life. •Depending on the roles, bonds and the structure of the amino acid, the proteins in the cell membrane play the role of: ochannels to facilitate diffusion and to transfer molecules according to electrical and chemical qualities oand as transporters (they bind with glucose molecules to transport them to the other side of the membrane). oorganic catalysts in human anatomy •They also go on to effect the world by being able to influence the nature of enzymes. • • • • Large polymers are made in a process called dehydration synthesis. They are constructed by linking small monomers (building blocks) together. Each time two monomers are linked, a molecule of water must be removed. Dehydration refers to the removal of water and synthesis refers to the linking of the monomers together. Small monomers are made in process called hydrolysis. Basically, large polymers are broken back down into small monomers by the addition of water. Hydro stands for the addition of water and lysis means to break. A monomer is a molecule that is able to bond in long chains. Polymer means many monomers (Poly means many). Monomers: Polymers: Carbohydrates: Monosaccharide Polysaccharide Oligosaccharide Disaccharide Lipids: Glycerol Fatty Acids Triglycerides Proteins: Amino Acids Polypeptides Carbohydrates consist of the elements carbon (C), hydrogen (H) and oxygen (O) with a ratio of hydrogen twice that of carbon and oxygen. In their basic form, carbohydrates are simple sugars or . These simple sugars can combine with each other( ) to form more complex carbohydrates (ex: Glucose). Monosaccharides are Monomers. The combination of two simple sugars is a (Ex: Sucrose). Disaccharides are Monomers. Those with a larger number are called starch). Polysaccharides are known as a Polymer. (Ex: Amylose Basically, a monosaccharide has just one ring, a disaccharide has two, and a polysaccharide has many. Fats and oils are made from two kinds of molecules: glycerol (a type of alcohol with a hydroxyl group on each of its three carbons) and three fatty acids joined by . A is a glyceride consisting of one fatty acid chain covalently bonded to a glycerol molecule through an ester (the bonding between fatty acids and glycerol that characterizes true fats) linkage. A is a glyceride consisting of two fatty acid chains covalently bonded to a glycerol molecule through ester linkages. Mono- and diglycerides are commonly added to commercial food products in small quantities. They act as emulsifiers, helping to mix ingredients such as oil and water that would not otherwise blend well. are formed by combining glycerol with three molecules of fatty acid. Basically, If only one long-chained carboxylic acid is bonded, it is called a monoglyceride. If only two are bonded, it is called a diglyceride. Only with three is it called a triglyceride. Proteins are made of Amino Acids, which are composed of carbon, nitrogen, oxygen, hydrogen and sometimes sulfur. Amino acids attach to each other by bonds. Two amino acids condense to form a joined together form a . to form covalent peptide , 3 form a and many If more than 100 amino acids attach together it is considered a protein. Primary is the term used to describe the order of the amino acids joined together to make the protein. If the “R” groups in the Amino Acid structure is replaced you would have the primary structure of a particular protein. Within the long protein chains there are regions in which the chains are organised into regular structures known as alpha-helices (alphahelixes) and beta-pleated sheets. These are the secondary structures in proteins. These secondary structures are held together by hydrogen bonds. In an alpha-helix, the protein chain is coiled like a looselycoiled spring. In a beta-pleated sheet, the chains are folded so that they lie alongside each other. The tertiary structure of a protein is a description of the way the whole chain (including the secondary structures) folds itself into its final 3-dimensional shape. The model shows the alpha-helices in the secondary structure as coils of "ribbon". The beta-pleated sheets are shown as flat bits of ribbon ending in an arrow head. The bits of the protein chain which are just random coils and loops are shown as bits of "string". A protein has quaternary structure if it is formed of 2 or more subunits (polypeptides). They are held together by various forces including hydrophobic interactions, H-bonds and ionic bonds.