pH Scale and Calculations - Gleneaglesunit1and2chemistry2012
advertisement

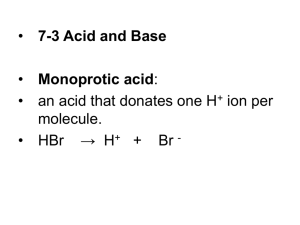
pH Scale and Calculations Chapter 14 pH Scale • We use this scale to measure the strength of an acid or base. • pH is defined as the –log[H+] • pH can use the concentration of hydronium ions or hydrogen ions. 7 Acid 0 Base pH Scale Zumdahl, Zumdahl, DeCoste, World of Chemistry 2002, page 515 14 pH of Common Substances Timberlake, Chemistry 7th Edition, page 335 Ionization of Water Occasionally, in water, a H+ is transferred between H2O molecules .. .. H:O: + :O:H .. H .. H water molecules .. H:O:H + .. .. + :O:H.. H hydronium ion (+) LecturePLUS Timberlake hydroxide ion (-) 5 Pure Water is Neutral Pure water contains small, but equal amounts of ions: H3O+ and OH- H2O + H2O H 3O + OH- H3O+ + OH- hydronium hydroxide ion ion 1 x 10-7 M 1 x 10-7 M 6 Ion Product of Water Kw Kw = [ H3O+ ] [ OH- ] = [ 1 x 10-7 ][ 1 x 10-7 ] = 1 x 10-14 7 pH of Common Substance More acidic More basic pH NaOH, 0.1 M Household bleach Household ammonia Lime water Milk of magnesia Borax Baking soda Egg white, seawater Human blood, tears Milk Saliva Rain Black coffee Banana Tomatoes Wine Cola, vinegar Lemon juice Gastric juice 14 13 12 11 10 9 8 7 6 5 4 3 2 1 0 [H1+] [OH1-] 1 x 10-14 1 x 10-13 1 x 10-12 1 x 10-11 1 x 10-10 1 x 10-9 1 x 10-8 1 x 10-7 1 x 10-6 1 x 10-5 1 x 10-4 1 x 10-3 1 x 10-2 1 x 10-1 1 x 100 1 x 10-0 1 x 10-1 1 x 10-2 1 x 10-3 1 x 10-4 1 x 10-5 1 x 10-6 1 x 10-7 1 x 10-8 1 x 10-9 1 x 10-10 1 x 10-11 1 x 10-12 1 x 10-13 1 x 10-14 pOH 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Acid – Base Concentrations concentration (moles/L) 10-1 pH = 3 pH = 11 OH- H3O+ pH = 7 10-7 H3O+ OH- OH- H3O+ 10-14 Timberlake, Chemistry 7th Edition, page 332 [H3O+] > [OH-] [H3O+] = [OH-] acidic solution neutral solution [H3O+] < [OH-] basic solution pH pH = -log [H+] Kelter, Carr, Scott, Chemistry A World of Choices 1999, page 285 Self-Ionization Of Water • Even the purest of water conducts electricity. This is due to the fact that water self-ionizes, that is, it creates a small amount of H3O+ and OH-. H2O + H2O H3O+ + OHKw = [H3O+][OH-] • Kw - ion product of water Kw = 1.0 x 10-14 at 25 oC • This equilibrium constant is very important because it applies to all aqueous solutions - acids, bases, salts, and non-electrolytes - not just to pure water. Self ionization reaction of water: O H H + H O O H H + H + O H 2 H2O H3O OH K w [H3O ] [OH ] 10 -14 Kw [H 3O ] [OH-] H (at 25C) pH and pOH • pH = - log[H3O+] pOH = - log[OH-] [H3O+] = 10-pH [OH-] = 10-pOH • pKw = pH + pOH = 14.00 • neutral solution: [H3O+] = [OH-] = 10 –7 M pH = 7.0 acidic solution: [H3O+] > 10-7 M pH < 7.0 [H3O+] < 10-7 M pH > 7.0 basic solution: Learning Check pH A. The [H3O+] of tomato juice is 1 x 10-4 M. What is the pH of the solution? 1) - 4 2) 4 3) 8 B. The [OH-] of an ammonia solution is 1 x 10-3 M. What is the pH of the solution? 1) 3 2) 11 LecturePLUS Timberlake 3) -11 14 Solution pH4 A. pH = - log [ 1 x 10-4] = -(- 4) = 4 B. [H3O+] = 1 x 10-11 pH = - log [ 1 x 10- 11] = -(- 11) = 11 LecturePLUS Timberlake 15 Learning Check pH5 The pH of a soap is 10. What is the [H3O+] of the soap solution? 1) 1 x 10 - 4 M 2) 1 x 1010 M 3) 1 x 10 - 10 M LecturePLUS Timberlake 16 Solution pH5 The pH of a soap is 10. What is the [H3O+] of the soap solution? [H3O+] = 1 x 10-pH M = 1 x 10-10 M LecturePLUS Timberlake 17 pH on the Calculator [H3O+] is 4.5 x 10-6 M pH = -log(4.5 x10-6 ) = 5.35 18 Learning Check pH6 A soap solution has a [H3O+] = 2 x 10-8 M. What is the pH of the solution? 1) 8 2) 7.7 3) 6 LecturePLUS Timberlake 19 Solution pH6 A soap solution has a [H3O+] = 2.0 x 10-8 M. What is the pH of the solution? B) = 7.7 20 Calculating pH from pOH • The concentration of [OH–] in a cleaning product was found to be 10–3 mol L–1. • Find the pH of the solution. Practise • (a) A 0.01 mol L–1 solution of HNO3 is prepared. Calculate the pH of the • solution. • (b) A 0.005 mol L–1 solution of H2SO4 is prepared. Assuming complete • ionisation, calculate the pH of the solution. Sample Problem: Calculating the pH of a dilution • 50 mL of a solution of HCl of pH 2 is diluted so that the new pH is 5. What • volume of water was added?