Preliminary Chemistry The Chemical Earth Weekly Work
advertisement

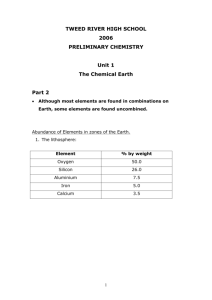
Preliminary Chemistry The Chemical Earth Weekly Work Sheet Term:1 Week: 6 Hi all, This week we are looking at the periodic table and how elements are arranged on the periodic table. We will examine the ideas of why some elements are found as pure elements and why some are very reactive and always found combined with other elements. Remember any questions or problems please do not hesitate to contact by email at: kerrilee.logan @det.nsw.edu.au or by phone at Ardlethan Central School 69782046. Talk soon, Kerrilee Logan Syllabus area: 2. Although most elements are found in combinations on Earth, some elements are found uncombined Students learn to: explain the relationship between the reactivity of an element and the likelihood of its existing as an uncombined element classify elements as metals, non-metals and semi-metals according to their physical properties account for the uses of metals and non-metals in terms of their physical properties construct word and balanced formulae equations of chemical reactions as they are encountered Students: plan and perform an investigation to examine some physical properties, including malleability, hardness and electrical conductivity, and some uses of a range of common elements to present information about the classification of elements as metals, non-metals or semi-metals analyse information from secondary sources to distinguish the physical properties of metals and non-metals process information from secondary sources and use a Periodic Table to present information about the classification of elements as: - metals, non-metals and semi-metals solids, liquids and gases at 25˚C and normal atmospheric pressure Your work: Refer to Chapter 1: Mixtures, Elements and Compounds in the Earth, read specifically section 1.23, 1.24, 1.25, 1.26 and 1.27. Make a summary of the ,ajor points in this information Obtain a copy of the periodic table and colour code it to reflect where metals, nonmetals and semi-metals occur on the periodic table Develop a table to show the difference between metals and non-metals in terms of : malleability, hardness, electrical conductivity, state at room temperature Complete worksheets attached/faxed Practical work: Work with your teacher to: plan and perform an investigation to examine some physical properties, including malleability, hardness and electrical conductivity, and some uses of a range of common elements to present information about the classification of elements as metals, non-metals or semi-metals (refer to faxed practical sheet if you need ideas. Link Lesson Monday: Atomic Structure Thursday: Periodic Table Have a great week, Kerrilee Logan