Acid Rain - Atmospheric and Oceanic Science

Acid Rain
RUSSELL R. DICKERSON
Sandstone portal
Figure on Herten
Castle in Ruhr district of Germany.
Sculpted 1702; photographed in
1908.
Same sandstone portal figure photographed in
1969.
VII.ACID DEPOSITION
See Turco, Ch. 9
Finlayson-Pitts & Pitts, Part 6
Seinfeld & Pandis Ch. 20
Introduction
The industrial revolution greatly increased the amount of acid in the atmosphere. These acids accelerate the rate of “weathering” of both natural and man-made materials. (Figures of sandstone Madonna). Today we will discuss what acid deposition is, what its effects are, the chemistry and physics of formation and transport, and who is responsible for it.
History
As early as 1852, R. A. Smith analyzed rain that near the industrial city of Manchester, England and found that urban aerosol particles tend to be composed primarily of sulfuric acid, but as the air is transported away from sources over more rural areas, the acid is neutralized by absorption of ammonia.
urban → suburban → rural
H₂SO₄ + NH₃ → (NH₄)HSO₄ (+NH₃) → (NH₄)₂SO₄ sulfuric acid → ammonium bisulfate → ammonium sulfate
Throughout the early part of the twentieth century, European scientists documented the sources and effects of atmospheric acids. It was not until
1958 that acidity of precipitation in the US was characterized (Junge and
Werby, 1958)
Definitions wet deposition dry deposition occult deposition
Effects
Soils
Soils (Figure) have colloidal molecules (clay particles) that have a layer of negative charge. They hold positively charged cations such as ,
4
Al³⁺, K⁺, Mg²⁺, and Ca²⁺. Some of these cations are essential plant nutrients (K⁺, Mg²⁺, Ca²⁺), while Al³⁺ is toxic. Hydrogen ions from acid deposition replace these cations on the outer layer of colloidal molecules.
The metal ions are then dissolved and leached into solution and can be washed away from the soil and into surface or ground water. Soil fertilitiy is reduced and aluminum ions can replace calcium in the fish’s gills.
Acidity can also come from oxidation (weathering) of sulfur-rich mine tailings, and from nitrification of ammonium.
NH
4
(bio)
NO
2
(N
2
O, NO, N
2
)
(bio)
NO
3
A soils capacity to exchange base ions for those in solution ( cation exchange capacity ) is a measure of potential fertility. The impact of acids on soil fertility depends on the structure and composition of the clays in the soil. Maryland has serveral geological regimes with varying sensitivities to acid deposition (Figure).
Forests can be especially sensitive to nutrient loss. In Europe in 1993 abou ta quarter of the trees have died or are more than 25% defoliated.
This “ Waldsterben ” or “forest death” has been attributed at, least in part, to environmental degradation from a combination of acid deposition, ground-level ozone, and excess nutrification, primarily nitrogen. In the
US, loss of forests has been so dramatic, although several species including ash and oak are sensitive to acidification of soils.
Lakes and Streams
2H
Ca
2
CO
3
2
CO
2
Ca
2
The sensitivity surface waters depends critically on their neutralizing or buffering capacity. Alkaline materials such as CaCO₃, and MgCO₃ can neutralize acids.
The surface of the American Midwest is predominantly limestone
(CaCO₃), and lakes and streams have high neutralizing capacity. In the
East granite dominates; soils and surface waters lacking buffering capacity, are highly sensitive to acidification.
Salt Waters
The Ocean is strongly pH buffered, has a high sulfate content naturally, and is relatively insensitive to “acid rain.” Over much of the ocean, however, nitrate is the limiting nutrient for biological activity.
Addition of nitrogen from the atmosphere can increase primary productivity, and the consequences reverberate throughout the ecosystem. The Chesapeake Bay is particularly sensitive to excess nutrients. Additional nitrate or ammonium from atmospheric deposition to the Bay or its watershed contributes to the problem of excess eutrophication. Nitrate allows thick beds of aquatic layers of the waters. Loss of higher animals follows.
Materials
The Taj Mahal, the friezes on the Parthenon, the Madonna in Herten,
Germany, and the Lincoln Memorial are made of marble. Marble, a particular crystalline form of calcite (CaCO₃), and sandstone (grains of sand held together by calcite), are subject to attack by sulfuric acid.
CaCO
3
H
2
SO
4
CaSO
4
CO
2
CaSO₄ is gypsum, which is 100 times more soluble than CaCO₃. Many priceless historic structures have been lost to acid deposition.
On a more pragmatic note, the rate of corrosion of galvanized (zinc coated) steel is 0.62 um/yr in the Adirondacks, 1.01 in Washington, DC, and 1.47 in Stubenville, OH.
Health
Covered already in Lectures 1 and 9.
Origins
Primarily power generation and ore smelting (pie charts).
For example nickel is mined as nickel sulfide, NiS. In smelting, it is heated in air (Sudbury, Canada).
NiS
O
2
Ni
SO
2
The molecular weight of nickel is 57 g/mole, so smelting produces more than a ton of SO₂ for each ton of nickel produced.
d) Formation and Composition
Gas Phase production of nitric acid:
OH + NO₂ + M → HNO₃ + M
Aqueous phase production of nitric acid:
NO₂ + O₃ → NO₃ + O₂
NO₃ + NO₂ + M = N₂O₅ + M
N₂O₅ + H₂O(l) → 2HNO₃(aq)
This process is important only at night, and when air temperatures are low because the formation of N₂O₅ is reversible, and the equilibrium coefficient is highly temperature dependent. Also, NO₃ is rapidly photolyzed by visible radiation.
NO₃ + hv → NO₂ + O
Gas Phase production of sulfuric acid:
OH + SO₂ + M → HOSO₂ + M
Aqueous phase production of sulfuric acid:
SO₂ + H₂O₂ → H₂SO₄
Lifetime and Transport
Wet vs dry deposition
Problem Solving
1.
European community requirements: 2.0 g/m3 and 0.4 g/m3 if coal is
0.5 to 7% S by weight, can such goals be met by buying low sulfur coal??
SO 2
2.
Calculate the pH of a solution of 10 μM and 10 μm NO
3
3.
From the mean pH of 4.1, and the assumption of equal molar nitrate and sulfate, estimate the molarity of nitric and sulfuric acids in
College Park.
4.
Estimate the rate of dry deposition for a give PBL height, and concentration of H₂SO₄ and HNO₃.
5.
If rainout plus washout is 50% efficient, what would have to be precipitation frequency for there to be equivalent amounts of wet and dry acid deposition?
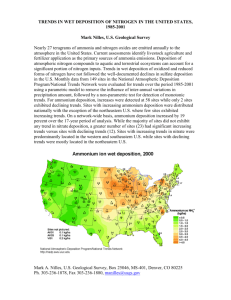
Average Precipitation pH Over the
United States
CHEMICAL COMPOSITION
OF PRECIPITATION
PATTERNS OF SO
2
AND NO
x
EMISSIONS IN EASTERN U.S.
SO
2
: coal-fired power plants
NO x
: transportation, power plants
4 18
NO
3
OH
(CH
3
)
2
S
(DMS) t =
1.0d
22
Phytoplankton
GLOBAL SULFUR BUDGET
(flux terms in Tg S yr
-1
)
cloud
42
SO
2 t =
1.3d
OH
8
H
2
SO
4
(g)
SO4 2t =
3.9d
10
64
Volcanoes Combustion
Smelters dep
27 dry
20 wet dep
6 dry
44 wet
GLOBAL SULFUR EMISSION TO
THE ATMOSPHERE
1990 annual mean
Chin et al. [2000]
AREAS (IN BLACK) WITH
LOW
ACID-NEUTRALIZING
CAPACITY
TRENDS IN U.S. EMISSIONS
OF NO
x
AND SO
2
TRENDS IN SULFATE AND
NITRATE WET DEPOSITION
DEPLETION OF BASE CATIONS
FROM ACID RAIN
(Hubbard Brook Experimental
Forest, New Hampshire)
QUESTIONS ON ACID
RAIN
1. A dinosaur extinction theory suggests that massive heating of the Earth's atmosphere in the path of a falling asteroid would have led to extremely acid rain, killing the land vegetation and hence the dinosaurs' food supply. What acid would have been involved?
2. Nitrate deposited by acid rain can fertilize the biosphere. Explain why.