Theory notes for week 5- 4March 2014
advertisement

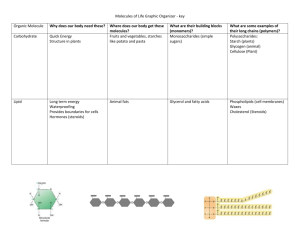
NSWTSCN312A- Investigating biological processes Theory notes for Week 6 (4 March 2014) Please pre-read these notes before class, they will reinforce the concepts that you will cover in class this week. Youtube videos may also be posted on the wiki to help you understand these concepts. Biochemical molecules: All cells are made of the same basic biochemical substances, no matter whether it is a liver cell or cheek cell, or whether it belongs to an insect or a human. Biologically important molecules are grouped into two major classes- organic and inorganic compounds. Inorganic compounds are those that are not produced by living organisms, while organic compounds are mostly produced by living organisms and contain one or more carbon atoms in their molecules. Organic compounds include: Carbohydrates (cellulose, starch and sugar) Lipids (fats, waxes and steroids) Proteins (eg haemoglobin, hormones, keratin, muscle fibres) Nucleic acids (which make up DNA & RNA) But first…….some basic chemistry: All things are made up of atoms. In the world there are about 90 different types of naturally occurring atoms or ‘elements’. The most abundant element is Oxygen (O), followed by Silicon (Si). Carbon (C), Hydrogen (H), Nitrogen (N), Sulfur (S) and Phosphorous (P) are also elements. The letters shown in brackets are the chemical symbols for the different elements. Atoms can combine together to form molecules. When atoms of different elements combine (bond together) they form molecules of a compound. Water is a compound- it is made of 2 Hydrogen atoms bonded with 1 Oxygen atom. Hence it’s chemical formula is H2O. Water is an inorganic compound. The type of compound formed and it’s basic structure is a result of what types of atoms it contains and how these combine. Have a look at the examples below to see the interesting shapes some molecules can form. Substance Water Chemical formula H2O Carbon dioxide CO2 Oxygen (gas) O2 Filename: Theory notes for week 6- 4 March 2014 Document Owner: General Education Cooma Queanbeyan Campus Atomic structure Page 1 of 11 Glucose C6H12O6 Urea CO(NH2)2 Table salt NaCl Molecules contain energy: All molecules contain energy. For example, molecules are moving all the time and so possess kinetic (or movement) energy. This is called Brownian motion. The amount of kinetic energy possessed by molecules varies depending on what phase state (solid/liquid or gas) a substance is in. For example in a solid molecules are densely packed, and so don’t move much (this is why they hold their shape so well). In a liquid however, particles slide over each other (like marbles in a bag), which is why liquid has fluid properties. In a gas, the molecules are airborne (like bees in a bottle) and this is why gases spread through the air readily. Atoms are held together by chemical bonds. It requires energy to hold a bond together(this is stored chemical energy). When a bond is broken (ie when parts of a molecule split up) the energy held in a bond is released. This allows organisms to gain energy when they break down complex molecules such as carbohydrates. This energy is called chemical stored energy. Water- the most common biological compound: Water is the most abundant molecule found in living organisms, which contain 70-90% water by weight. It is an inorganic compound. Water has special properties which make it an ideal medium for living organisms and allows it to be a carrier for nutrients, oxygen, and waste products to and from cells. The latter is linked to the fact that water will readily dissolve some substances, such as salts, O2 and CO2. The fact that water can dissolve many substances has earned it the name as the ‘universal solvent’. Substances (solutes) that dissolve readily in water are called hydrophilic and this is due to the fact that they are polar molecules. Polar molecules attract other polar molecules and so they can intermix readily, such as when salt dissolves in water. Substances that do not dissolve in water (such as fats) are hydrophobic and this is due to the fact that they are non-polar molecules. Non-polar molecules are repelled by polar molecules and so these non-polar molecules will tend to form globules in the presence of polar molecules such as water. This is why oil and water does not mix. Organic compounds: Organic compounds contain carbon and have been made by a biological organism. Carbon is a special element in that its atoms are able to form strong bonds between other carbon molecules to form rings and chain type molecular structures. In combination with other types of atoms, there is a wide range of variation in organic molecule types that can be formed and this is why organic compounds are suited to the complexity required for living things. You will see how complex these molecules can be below. Filename: Theory notes for week 5- 4 March 2014 Document Owner: GEAP Cooma Page 2 of 11 There are standard laboratory tests which can be used to determine the presence of carbohydrates/ proteins and lipids in a sample. You will research how to do these tests for homework as part of your worksheet. Carbohydrates: Carbohydrates are the most abundant organic compound in nature. Carbohydrates are a source of immediate as well as stored chemical energy for living organisms. They also form structural components of roganisms and can combine with other molecules such as proteins. Carbohydrates are composed of carbon, hydrogen and oxygen atoms. These are bound together to form a variety of different molecules all of which are arranged in either in chains or rings of carbon as their basic molecular structure. There are three main groups of carbohydrates: Monosacharides – these are simple sugars and have the smallest molecular structre out of all the carbohydrates. They are sweet tasting and can be made up of 3,5 and 6 carbon atoms. The most common of which is glucose (C6H12O6), which has 6 carbon atoms, 12 Hydrogen and 6 Oxygen. It is made during photosynthesis. More complex starches can have the glucose molecule as their base unit. A glucose molecule is shown below: Disaccharides- consist of two monosaccharides bonded together. Sucrose, maltose and lactose are exaples of common disaccharides. Sucrose is made up of glucose and fructose molecular subunits, and is how plants transport glucose from leaves to other plarts of a plant. Lactose (a sugar contained in mammalian milk) is made up of glucose and galactose subuints. A sucrose molecule is shown below: Polysaccharides- consists of many monosaccharides joined in long linera or branched chains, called polymers. They can be made of a single type of monosaccharide or maore than one type. They are usually tasteless, insoluble copounds that serve to store energy or provide structural purposes. For example starch is made up of many hundredsof glucose units linked in a chain, some of which also can have a branhed structure. This long molecules tends to ‘coil up’ when in water and makes the molecule insoluble in water. To access the glucose in this molecules, enzymes must be employed to break it down. Cellulose is a polysaccharide that is used as a structural compoenent in plants (50% of wood or cotton is nearly pure cellulose). Cellulose is also made up of chains of glucose molecules, but these are bonded together in a Filename: Theory notes for week 5- 4 March 2014 Document Owner: GEAP Cooma Page 3 of 11 different way. The result is a molecule which cannot be readily digested by animals because it requires a different enzyme (cellulase) to do so. Some bacteria, proptpzoa and funig secrete this enzyme. A starch unit is shown below, it is usually made up of between 300-600 of these units in a long chain: Lipids: Lipids are a groups which contain fats, waxes, steroids and phospholipids. Fats- are chain like molecules which have a backbone unit of glycerol (containing three carbon atoms, 6 Hydrogen and 3 Oxygen) attached to which cahins of fatty acids are attached. Fatty acid molecules are hydrocarbon chains to which a special molecular grouping called a carboxyl (-COOH) is attached. Because there is a lot of energy stored in the bonds of these molecules, they can serve as efficient storage units for chemical energy. Saturated fats are animals fats such as butter, lard and animal fat and are usually solid at room temperature. Unsaturated fats such as canola, olice and corn oils are liquid at room temperature. On a molecular level, a saturated fat molecule has every available space on the molecule bonded with hydrogen atoms. A fat molecule is shown below, note the glycerol to the left and the carboxyls linking the hydrocarbon cahin tails to the glycerol molecule: Waxes- have a similar structure to fat molecules, but they have a long chain alcohol as a backbone sub-unit to which hydrocarbon tails are joined. This material forms a protective coating on the outside of animals and plants. Phospho lipids- have a glycerol base unit to which two fatty acids are attached and also include a phosphate group. This phosphate group gives the head of the molecule a negative charge (it becomes polar). The fatty acid tail is non-polar, as shown in the diagram below. Filename: Theory notes for week 5- 4 March 2014 Document Owner: GEAP Cooma Page 4 of 11 This gives rise to the ability for this molecule to form the cell membrane in which the polar and nonpolar ends of the molecules align in a phospholipids bilayer structure, as shown below. Steroids- Have a different structure to most fats (based on rings of 4 carbons), but are included in the group because they are fat soluble. Steroids are important in animals and found in the heart, liver and blood vessels. Some hormones are also steroids. Cholesterol is a common steroid and its function in the body is to act as the starting point for the synthesis of several hormones. Cholesterol, being insoluble in water, may precipitate from bile in the gall bladder and form pebble like gall stones. It can also form plaques in arteries and cause high blood pressure and increase risk of heart attacks/strokes. Proteins: Proteins are structurally varied molecules which perform a wide range of functions including: enzymes for metabolism, proteins for structure, movement, metabolism, nutrition and transport, antibodies and toxins for defence and attack and hormones for regulation of body functions. Proteins contain Carbon, Hydrogen, Oxygen, Nitrogen and Sulfur. The building blocks of proteins are amino acids, of which there are 20 different types. The number of possible combinations of amino acids, and hence the variety of proteins is enormous. Amino acids combine to form polypeptide chains. A protein may be made up of one or more polypeptide chains. These chains can cross link (bond together) with several other chains, resulting in complex and twisted shapes of protein molecules. The diagram below show the stages of protein synthesis. Filename: Theory notes for week 5- 4 March 2014 Document Owner: GEAP Cooma Page 5 of 11 The shape of the protein molecule influences the nature of the protein. For example, fibrous proteins (such as keratin, collagen and elastin), polypeptide chains are arranged parallel to one nother forming long fibres or sheaths. These are generally non-soluble. In globular proteins, the polypeptide chains are folded and twisted into globular structure. This helps make them soluble and includes proteins such as horomones and enzymes. The image below shows a straight protein molecules as found in collagen (connective tissue) and a globular protein found in haemoglobin. Nucleic acids: Nucleic acids are the molecules that make up DNA and RNA of a cell. DNA is the genetic material of a cell, while RNA carries the coded information to make proteins. Nucleic acids are chains of repeating nucleotide units or poly nucleotides. Nucleotides are made up of a 5 carbon sugar, a phosphate group and a nitrogenous base. There are five different nucleotides (cytosine, uracil, thymine, adenine and guanine) which combine in a double stranded, coiled ladder like structure to form DNA. This is shown in the diagram below, where you can see the carbon chains forming the sides of the ladder and the nitrogenous base ends of the nucleotides meeting in the middle of the molecule to form the ‘rungs of the ladder’. Filename: Theory notes for week 5- 4 March 2014 Document Owner: GEAP Cooma Page 6 of 11 The RNA nolecule is similar except it only contains only one strand of molecules as shown in the diagram below. Enzymes help to catalyse biochemical reactions: As we saw in the last lesson, biochemical reactions enable plants to make food (photosynthesis) and both plants and animlas to extract energy from it (respiration). On a cellular level, chemical recations therefore enable the organisms function to be carried out. However, one problem is that many chemical reactions normally proceed far too slowly to be of any use to organisms, especially at the temperatures at which most organism functions. Enzymes (usually made up of prteins) are biological catalysts which work to Filename: Theory notes for week 5- 4 March 2014 Document Owner: GEAP Cooma Page 7 of 11 accelerate the biological reactions in cells. An animal cell can contain up to 400 different enzymes. Some enzymes are common to many different cells, while others are specific to cells carrying out different functions. Most enzymes are very specific in the way they function, bringing together two types of molecules to make a specific chemical recation occur. Enzyme activity is regulated so that appropriate amount of products are made up at the required rate. pH and temperature is also important to regulate the rate of a reaction and so these must be regulated within a cell. ATP as an energy carrier: ATP (or adenosine triphosphate) is a molecule produced by respiration. In the ATP molecules, a lot of energy is held in the bond between the phosphate atoms bonded to the rest of the molecule. Breakdown of the molecule from ATP to ADP (adenosine diphosphate) releases a phosphate atom and releases the energy stored in the bond which can then be used by the cell to drive non-spontaneous (ie those that wouldn’t occur on their own accord) reactions. Movement of substances across membranes: All living cells are surrounded by an aqueous medium. For an animal or plant cell this is the intercellular (or interstitial) fluid. The composition of the cell is different to that of the outside fluid. The stability of the cellular environment (homeostasis) must be maintained in the face of changes in both the inner and outer cell environment. The inner cell environment changes as metabolism occurs, for example oxygen is used up and carbon dioxide is produced. In order for a cell to remain healthy, concentrations of substances dissolves in the cell fuid must remain in defined limits. This means various organic (eg glucose) and inorganic (eg O2) substances must enter the cell so they can be used for cell processes and wastes (such as CO2) or other substances especially made for export must leave the cell. Cells are separated from this fluid by their cell membrane, which is selectively permeable to let in and out certain substances needed for the functioning of the cell. Without the correct functioning of this membrane the cellular processes would fail and the cell would die. The cell membrane is permeable to substances such as water, O2, N2, CO2, urea and ethanol. It is not particularly permeable to molecules such as glucose, sucrose, charged ions (Na+/K+ etc) and macro molecules such as proteins and nucleic acids). This movement therefore occurs via passive transport (eg osmosis and diffusion letting substances permeate across the membrane) and active transport (for those substances that cannot permeate the cell wall). Diffusion The simplest mechanism by which substances move in and out of cells is diffusion. It is the passive movement of molecules along their chemical concentration gradient (ie between areas of higher and lower concentrations). It is the process by which molecules spread from areas of high concentration, to areas of low concentration. When the molecules are even throughout a space - it is called EQUILIBRIUM. Filename: Theory notes for week 5- 4 March 2014 Document Owner: GEAP Cooma Page 8 of 11 It is called passive movement because it requires no renergy apart from the inherent movement energy of the particles themselves. This is why diffusion occurs faster at higher temperatures. Once concentrations have equalised, there will be no further net movement. The rate of diffusion results from permeability of a membrane to that substance and the diffusive force of that molecule. This is the mechanism by which O2 and CO2 enters the cell, by which alcohol rapidly enters the bloodstream and anaesthetics are absorbed via the lungs. Chemical pesticides, such as DDT are highly soluble in lipids and are thus easily able to penetrate cells and accumulate. Osmosis Water is the most abundant substance in a cell and determines a cell’s volume. It too must be regulated, because if too much water enters the cell, it would dilute the other substances in the cytosol (cell fluid) to support efficient metabolism. If too much water leaves the cell, the same occurs. Therefore water movement across cell membranes must be controlled and this occurs via osmosis. Osmosis is the process whereby water moves across a semi-permeable membrane. Water will move in the direction where there is a higher concentration of solute (and hence a lower concentration of water) until the two equalise as shown in the diagram to the right. A simple rule to remember is: salt sucks! Salt is a solute, when it is concentrated inside or outside the cell, it will draw the water in its direction. This is also why you get thirsty after eating something salty. Lets look at how osmosis works in solutions of different concentrations in and outside of the cell. Isotonic Solutions If the concentration of solute (salt) is equal on both sides, the water will move back in forth but it won't have any result on the overall amount of water on either side. "ISO" means the same. Hypotonic Solutions The word "HYPO" means less, in this case there are less solute (salt) molecules outside the cell, since salt sucks, water will move into the cell. The cell will gain water and grow larger. In plant cells, the central vacuoles will fill and the plant becomes stiff and rigid, the cell wall keeps the plant from bursting. In Filename: Theory notes for week 5- 4 March 2014 Document Owner: GEAP Cooma Page 9 of 11 animal cells, the cell may be in danger of bursting, organelles called CONTRACTILE VACUOLES will pump water out of the cell to prevent this. Hypertonic Solutions The word "HYPER" means more, in this case there are more solute (salt) molecules outside the cell, which causes the water to be sucked in that direction. In plant cells, the central vacuole loses water and the cells shrink, causing wilting. In animal cells, the cells also shrink. In both cases, the cell may die. This is why it is dangerous to drink sea water - its a myth that drinking sea water will cause you to go insane, but people marooned at sea will speed up dehydration (and death) by drinking sea water. This is also why "salting fields" was a common tactic during war, it would kill the crops in the field, thus causing food shortages. Importance of Surface Area:Volumer (SA:vol) ratio: The surface area to volume ratio is an important factor for the rate at which chemical reactions in cells or the process of diffusion etc will proceed. For example a cell with a small SA:vol ration (ie has a small surface area and large volume) will be able to diffuse oxygen at a slower rate than one with a large SA:vol ratio (ie has a large surface area and small volume). In biology the ratio between the surface area and volume of cells and organisms has been utilised to help organisms adapt to their environment. For example, many aquatic microorganisms have increased surface area to increase their drag in the water. This reduces their rate of sink and allows them to remain near the surface with less energy expenditure. An increased surface area to volume ratio also means increased Filename: Theory notes for week 5- 4 March 2014 Document Owner: GEAP Cooma Page 10 of 11 exposure to the environment. The many tentacles of jellyfish and anemones are the result of increased surface area for the acquisition of food. Greater surface area allows more of the surrounding water to be sifted for food. Individual organs in animals are often based on the principle of greater surface area. The lung is an organ with numerous internal branchings that increase the surface area through which oxygen is passed into the blood and carbon dioxide is released from the blood. The intestine has a finely wrinkled internal surface, increasing the area through which nutrients are absorbed by the body. This is done to increase the surface area in which diffusion of oxygen and carbon dioxide in the lungs and diffusion of nutrients in villi of the small intestine can occur. Increased surface area can also lead to biological problems. More contact with the environment through the surface of a cell or an organ (relative to its volume) increases loss of water and dissolved substances. High surface area to volume ratios also present problems of temperature control in unfavorable environments. This is why babies are more vulnerable to heat loss and are affected much more by burns to the body compared to adults. Organisms have adapted to overcome the biological problems of SA:vol ratio. Smaller animals in colder environments have higher metabolic rates tso they can make more body heat to keep warm. Circulatory systems in animals allow transport of substances to cells to make up for reduced SA:Vol ratio in a larger organism. Filename: Theory notes for week 5- 4 March 2014 Document Owner: GEAP Cooma Page 11 of 11