5th lecture on the Kolmogorov-Johnson-Mehl
advertisement

1
Objective
Bio ex.
Microstructure-Properties: II
The KJMA Equation
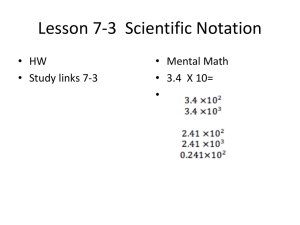
Notation
Assumptions
Derivation
Plots
27-302
Lecture 5
Fall, 2002
Prof. A. D. Rollett
2
Materials Tetrahedron
Processing
Performance
Objective
Bio ex.
Notation
Assumptions
Derivation
Plots
Microstructure
Properties
3
Objective
Objective
Bio ex.
Notation
Assumptions
Derivation
Plots
• The objective of this lecture is to introduce
the concept of phase transformation kinetics
as described by the Kolmogorov-JohnsonMehl-Avrami equation.
• Part of the motivation for this lecture is to
prepare the class for a Lab on the
crystallization of glass-ceramics.
4
References
•
•
Objective
Bio ex.
Notation
Assumptions
Derivation
Plots
•
•
•
•
•
Phase transformations in metals and alloys, D.A. Porter, & K.E.
Easterling,Chapman & Hall, 0-412-45030-5, 669.94 P84P2: page
289.
Kolmogorov, A. (1937). “A statistical theory for the recrystallization of
metals.” Akad. nauk SSSR, Izv., Ser. Matem. 1: 355.
Johnson, W. and R. Mehl (1939). “Reaction kinetics in processes of
nucleation and growth.” Trans AIME 135: 416.
Avrami, M. (1939). “Kinetics of Phase Change. I: General Theory.” J.
Chem. Phys. 7: 1103.
Avrami, M. (1940). “Kinetics of Phase Change. II: TransformationTime relations for random distribution of nuclei.” J. Chem. Phys. 8:
212.
Avrami, M. (1941). “Kinetics of Phase Change. III: Granulation,
Phase Change an Microstructures.” J. Chem. Phys. 9: 177.
Anderson, W. and R. Mehl (1945). “Recrystallization of Al in terms of
the rate of nucleation and growth.” Trans. AIME 161: 140.
5
Transformation Kinetics
Objective
Bio ex.
Notation
Assumptions
Derivation
• The kinetics of transformation are typically
described by a standard equation known as
the Kolmogorov-Johnson-Mehl-Avrami
equation, named after the individuals who
derived it.
• The characteristic of the kinetics is that of
the “S-curve”, i.e. slow at first, then
accelerating, then decelerating.
Plots
f
1 exp kt
n
6
Transformation kinetics are universal
Objective
Bio ex.
Notation
Assumptions
Derivation
Plots
• The kinetics of transformation are universal.
• Consider this example of the kinetics of cell growth.
• High-Throughput Assay System for the Discovery
Of Anti-Bacterial Drugs
1J.
Bruce Pitner, 2Mark R. Timmins, 2Maurice Kashdan, 3Mandar Nagar, and 3David T. Stitt,
Technologies 21 Davis Drive, Research Triangle Park, NC 27709, 2BD Biosciences, Two Oak Park,
Bedford, MA 01730, 3BD Biosciences, 250 Schilling Circle, Cockeysville, MD 21030; Presented at AAPS
- New Orleans, LA November, 1999
1BD
7
Cell culture growth kinetics
Objective
Bio ex.
Notation
Assumptions
Derivation
Plots
Note the “S-curve” kinetics
8
Derivations
Objective
Bio ex.
Notation
Assumptions
Derivation
Plots
• First, a remark on derivations.
• The objective of a derivation is to build a
(mathematical) bridge between basic concepts
(axioms in math) and a result (generally, an
equation, with parameters or variables
corresponding to physical quantities).
9
Transformation types
Continuous nucleation:
nuclei added
during transformation.
Objective
Bio ex.
Notation
Assumptions
Derivation
Plots
Site Saturated:
all nuclei present
at t=0.
Cellular:
recrystallization,
for example.
Kinetics same as for
site saturated case.
10
KJMA notation
Objective
Bio ex.
Notation
Assumptions
Derivation
Plots
• The central idea in the derivation of the KJMA
equation is to focus on the increment in the (volume)
fraction transformed and to relate it to the current
value of the fraction transformed.
• Notation:
f
fraction transformed
t
time
t50% time required for 50% transformation
r
radius
V volume
v growth rate (speed)
t incubation/delay time
N rate of nucleation, or, density of nuclei per unit
volume
11
Fraction transformed
• The relationship between volume and fraction
transformed is simple.
Fraction transformed = volume / total volume,
Objective
Bio ex.
or,
Notation
Assumptions
Derivation
Plots
f = V / Vtotal
• Similarly for area (2D), line (1D), etc.
12
KJMA: extended fraction
• To understand the concept of an extended fraction
transformed, imagine that each patch of new phase
can overlap with another one as they grow (ignore
the effect of impingement):
Objective
Bio ex.
V2
Notation
Assumptions
Derivation
Plots
V1
fext = (V1+V2)/Vtotal
f = (V1V2)/Vtotal
The true fraction transformed counts only the
actual volume transformed: the extended
fraction counts all volume as if no impingement
occurs.
“union of”
13
KJMA derivation: assumption
Notation
• There is one key assumption in the derivation of the
KJMA equation:
the nuclei are distributed randomly in space.
• This assumption allows us to make a quantitative
relation between the true increment in fraction
transformed, a fictitious or extended fraction
transformed and the current fraction:
Assumptions
df = dfext (1-f)
Objective
Bio ex.
Derivation
Plots
• Why does this work? The reason is that the volume
that each patch can grow into is decreased from the
total in proportion to the fraction that has already
transformed.
14
KJMA derivation
• The KJMA derivation is therefore a bridge between
the differential equation just stated and the final
equation that we use.
Objective
Bio ex.
Notation
Assumptions
Derivation
Plots
df = dfext (1-f)
f
1 exp kt
n
15
KJMA derivation: 1
Objective
Bio ex.
Notation
Assumptions
Derivation
Plots
• Step 1: define the differential equation (above).
• Step 2: describe the growth rate of an individual
patch/region.
• Example: 3D, isotropic growth, site saturated
nucleation:
V = 4π/3 r3(t) = 4π/3 (vt)3
• Step 3: multiply the individual volume by the
number density of nuclei, N:
fext = SVi /Vtotal = 4π/3 N (vt)3
• Step 4: obtain the extended fraction increment:
dfext = V/Vtotal = 4π Nv3 t2 dt
16
KJMA derivation: 2
• Step 5: insert the extended fraction increment into
the differential equation:
Objective
Bio ex.
df = dfext (1-f)
df = 4π Nv3 t2 dt (1-f)
Notation
Assumptions
Derivation
Plots
df/ (1-f) = (4π N v3) t2dt
• Step 6: collect the nucleation and growth terms into a
constant (which varies depending on the conditions
of nucleation and growth):
k = 4π/3 N v3
17
KJMA derivation: 3
• Step 7: solve the differential equation:
recognize that df = -1 * d(1-f), and that the fraction
transformed is zero at t=0, so that we are dealing
with a logarithmic solution.
Objective
- ln(1 - f ) = k t3
Bio ex.
Notation
Assumptions
Derivation
Plots
•
Re-arrange to obtain the final result:
f
1 exp kt
n
- general result
4
3 3 - site saturated,
1 exp Nv t
3D growth
3
18
KJMA solutions
Objective
Bio ex.
Notation
Assumptions
Derivation
• In general, the k value contains all the temperature
dependent terms because
thermal activation affects
the growth strongly through
boundary/interface mobility,
and because the nucleation
density depends very strongly
on driving force.
See P&E p269:
v = v(T) = v0 exp-(Q/RT)
Plots
• In general, the exponent n in the equation is related
to the geometry of the transformation.
19
“n” values
• Site saturated:
1D growth
2D growth
3D growth
1
2
3
Objective
Bio ex.
Notation
Assumptions
Derivation
Plots
• Continuous nucleation, constant nucleation rate:
1D growth
2
2D growth
3
3D growth
4
• CAUTION: you cannot always deduce the geometry
of transformation from the value of the exponent.
20
People
Objective
Bio ex.
Notation
Assumptions
Derivation
Plots
• Kolmogorov was a Russian mathematician who
work is much referenced in statistics. He worked
out this relation for the case of continuous
nucleation.
• Johnson was a graduate student at Carnegie Tech
under R.F. Mehl as his adviser. He studied
recrystallization in aluminum.
• Avrami was a chemist and worked out the most
general approach: his work is known in the
chemical engineering world.
• Porter & Easterling describe the equation but do not
explain it in detail. Other texts provide more detail.
21
KJMA plots
• A very useful way to analyze the kinetics of
transformation (e.g. recrystallization) is to plot the
quantity -ln(1-f) versus time on a double-logarithmic
plot. The slope of the line is then the exponent, n.
Objective
Bio ex.
log ln( 1 f ) log( k) nlog( t)
Notation
Assumptions
Derivation
Plots
[Humphreys]
n = slope = 2
22
Measurement
• The fraction transformed can be measured in
almost any conceivable way.
Objective
Bio ex.
Notation
Assumptions
Derivation
Plots
–
–
–
–
–
From micrographs
Hardness
Electrical resistivity
Optical properties
Calorimetry
KJMA derivation: diffusion controlled
growth
23
Objective
Bio ex.
Notation
Assumptions
Derivation
Plots
• Step 1: define the differential equation (above).
• Step 2: describe the growth rate of an individual
patch/region.
• Example: 3D, isotropic diffusion controlled growth,
site saturated nucleation:
V = 4π/3 r3(t) = 4π/3 {∆X0/(Xppt-Xe)}3 (√{Dt})3
= 4π/3 {∆X0/(Xppt-Xe)}3 (Dt)1.5
• Step 3: multiply the individual volume by the
number density of nuclei, N:
fext = SVi /Vtotal = N 4π/3 {∆X0/(Xppt-Xe)}3 (Dt)1.5
• Step 4: obtain the extended fraction increment:
dfext = V/Vtotal = N 2π {∆X0/(Xppt-Xe)}3 D1.5 √t dt
KJMA derivation: diffusion controlled
growth
24
• Step 5: insert the extended fraction increment into
the differential equation:
Objective
Bio ex.
Notation
Assumptions
Derivation
Plots
df = dfext (1-f)
df = 2π N {∆X0/(Xppt-Xe)}3 D1.5 √t dt (1-f)
df/ (1-f) = 2π N {∆X0/(Xppt-Xe)}3 D1.5 √t dt
• Step 6: collect the nucleation and growth terms into
a constant (which varies depending on the
conditions of nucleation and growth):
k = 4π/3 ND1.5 {∆X0/(Xppt-Xe)}3
KJMA derivation: diffusion controlled
growth
25
• Step 7: solve the differential equation:
recognize that df = -1 * d(1-f), and that the fraction
transformed is zero at t=0, so that we are dealing
with a logarithmic solution.
Objective
- ln(1-f) = 4π/3 N {∆X0/(Xppt-Xe)}3 (Dt)1.5
Bio ex.
Notation
Assumptions
Derivation
•
Re-arrange to obtain the final result:
f
1 exp kt n
f
3
- site saturated,
4
X0
1.5
1 exp N
Dt 3D diffusion
3 X X e
controlled growth
Plots
- general result
26
Fixed fraction transformed
• If we need to find the time for a fixed fraction
transformed, this is easily accomplished by
manipulation of the basic equation, e.g. for 10%
and 90% transformed.
Objective
Bio ex.
Notation
f
1 exp kt n f
1 exp kt n
Assumptions
0.1 1 exp ktn
0.9 1 exp ktn
Derivation
ln 0.9 ktn
ln 0.1 kt n
ln 0.9 1/ n
t
k
ln 0.11/ n
t
k
Plots
27
TTT Diagrams
Objective
Bio ex.
Notation
Assumptions
Derivation
Plots
• What is the connection between KJMA kinetics and
TTT diagrams?
• Answer: once you have defined the relevant
quantities in the KJMA equation, i.e. the nucleation
density and the growth rate (and exponent) as a
function of temperature, then you can calculate the
time required to achieve a certain fraction
transformed (previous slide).
• Armed with a set of times for a fixed fraction
transformed, draw the locus of points that is a curve
on the TTT diagram. Repeat for each volume
fraction of interest.