Cancer_biology_2009_CRC
advertisement

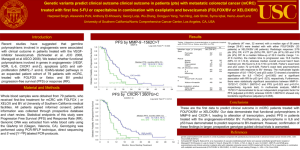
COLORECTAL CANCER Charles Lopez, M.D., Ph.D. Associate Professor of Medicine Hematology and Medical Oncology May 2009 Outline • Background. • Metastatic colorectal cancer. – Chemotherapeutic and biologic agents – Ongoing trials updates • Adjuvant colorectal cancer. – Current practice and ongoing trials COLORECTAL CANCER UPDATE 2007 Estimated US Cancer Cases* Men 766,860 Women 678,060 26% Breast 15% 15% Lung & bronchus Colon & rectum 10% 11% Colon & rectum Urinary bladder 7% 6% Uterine corpus Non-Hodgkin lymphoma 4% 4% Non-Hodgkin lymphoma Melanoma of skin 4% 4% Melanoma of skin Kidney 4% 4% Thyroid Leukemia 3% 3% Ovary Oral cavity 3% 3% Kidney Pancreas 2% 3% Leukemia 19% 21% Prostate 29% Lung & bronchus All Other Sites ONS=Other nervous system. Source: American Cancer Society, 2007. All Other Sites COLORECTAL CANCER UPDATE Epidemiology Lifetime Probability of Developing Cancer Men Women All sites 1 in 2 All sites 1 in 3 Prostate 1 in 6 Breast 1 in 8 Lung 1 in 13 Lung & bronchus 1 in 17 Colon and rectum 1 in 17 Colon & rectum 1 in 18 Urinary bladder 1 in 28 Uterine corpus 1 in 38 Lymphoma 1 in 46 Lymphoma 1 in 55 Melanoma 1 in 52 Ovary 1 in 68 Kidney 1 in 64 Melanoma 1 in 77 Leukemia 1 in 67 Pancreas Oral Cavity 1 in 73 Urinary bladder 1 in 88 Stomach 1 in 82 Uterine cervix 1 in 135 1 in 79 * For those free of cancer at beginning of age interval. Based on cancer cases diagnosed during 2000 to 2002. Source: DevCan: Probability of Developing or Dying of Cancer Software, Version 6.0 Statistical Research and Applications Branch, NCI, 2005. http://srab.cancer.gov/devcan Gastrointestinal Cancers in the United States Small intestine 2% Other digestive organs 2% Gallbladder 3% Esophagus 6% Anus 2% Liver 7% Colon 41% Stomach 8% Pancreas 13% Rectum 16% American Cancer Society. Cancer Facts and Figures 2006. Atlanta: American Cancer Society; 2006. Colorectal Cancer • Major public health problem in the US and worldwide • Worldwide, nearly 1,000,000 cases diagnosed each year • In the US, 130,000-140,000 cases diagnosed each year. • In the US with 50,000-60,000 deaths each year < 40% of CRC diagnoses are localized disease CRC = colorectal cancer. Colorectal Cancer Facts & Figures. 2005. American Cancer Society. Staging TNM Stage I I IIA IIB IIIA IIIB IIIC IV TNM Class T1N0M0 T2N0M0 T3N0M0 T4N0M0 T1-2N1M0 T3-4N1M0 TanyN2M0 TanyNanyM1 MAC A B1 B2 B3 C1 C2/C3 C1/C2/C3 D N2 denotes 4 or more regional lymph nodes (Need to sample at least 12 nodes). (Modified Astler-Coller and New TNM Staging) COLORECTAL CANCER UPDATE Epidemiology Stage I Stage II Stage III Stage IV Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 17 Regs Limited-Use, Nov 2006 Sub (1973-2004 varying), National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2007, based on the November 2006 submission. Risk Factors for Colorectal Cancer Genetic susceptibility FAP (risk approaches 100% by age 50) HNPCC (lifetime risk approaches 80%) Family history Adenoma (first degree) RR 1.72;11% lifetime risk CRC (first degree) RR 2.62; 16% lifetime risk Medical history Inflammatory bowel disease (pancolitis 8 years or left-sided colitis 15 years) (10-20% risk) Characteristics Age (91% of cases occur after age 50) Male sex (35% in men) Race/ethnicity (15% in African Americans) Obesity and diet (red meat, alcohol consumption) Smoking FAP = familial adenomatous polyposis; HNPCC = hereditary nonpolyposis colon cancer syndrome; CRC = colorectal cancer. Anderson FW, et al. J Natl Cancer Inst. 2002;94:1126-1133; Colorectal Cancer Facts & Figures. American Cancer Society; 2005; Levin B, et al. NCCN. v.1.2005; Lynch HT, et al. Cancer. 1995;76;2427-2433; Petersen GM, et al. Cancer. 1999;86(suppl):2540-2550; Winawer SJ, et al. Gastroenterology. 2003;124:544-560. Associations for Risk of Colorectal Cancer Dietary Factors: Dietary Fiber Dietary Fat Red Meat Alcohol Folate Calcium and vitamin D Non-Dietary Factors: Body Mass Index Physical Activity Hormone replacement Smoking Aspirin • HPFS, middle age men: BMI 25 kg/m2;Physical activity 15 MET-hours/week;Daily folate containing MVI;Alcohol < 15 g/day;Non-smoker;Red meat 2 servings/week (e.g.-- 3.1% of all men) – Eliminate 71% of all colorectal cancer! (95% CI, 33-92%) Colon Cancer: Multi-step Model of Carcinogenesis = biologic heterogeneity • • • • Cancers arise in polyps Develop over years Multiple molecular events Activation of oncogenes and loss of tumor suppressor genes Beart R. Clinical Oncology. Abeloff M, et al. Ed. 1998 Churchill Livingstone Evolution of a lethal cancer Jones S. et.al. PNAS 2008;105:4283-4288 ©2008 by National Academy of Sciences COLORECTAL CANCER UPDATE Screening Easily Overlooked Lesions Tied to Colon Cancer By Denise Grady An easily overlooked type of abnormality in the colon is the most likely type to turn cancerous, and is more common in this country than previously thought, researchers are reporting. lity in the colon is the most likely type to turn cancerous, and is more common in this country than previously thought, researchers are reporting. New York Times, March 5, 2008 COLORECTAL CANCER UPDATE Screening Flat Polyps Soetinko et al. JAMA. 2008;299(9):1027-1035 COLORECTAL CANCER UPDATE Screening Flat Polyps Soetinko et al. JAMA. 2008;299(9):1027-1035 Development of Systemic Treatments for Metastatic Colorectal Cancer 2004 Cetuximab + irinotecan (or alone) Bevacizumab + 5-FU-based chemotherapy Targeted regimens 5-FU dominates treatment for mCRC Investigations of 5-FU combination and schedules 1950 1960 1970 1980 1957 5-FU introduced Cytotoxic regimens 5-FU = 5-fluorouracil; LV = leucovorin; mCRC = metastatic colorectal cancer. Holen KD, Saltz LB. Lancet Oncol. 2001; 2:290-297; Venook A. The Oncologist. 2005;10:250-261. Targeted Therapies 1990 1996 Irinotecan (2nd line) 2000 Irinotecan + 5-FU/LV (1st line) 2000 2010 2006 Panitumumab 2004 Oxaliplatin + 5-FU/LV (1st line) 2002 Oxaliplatin + 5-FU/LV (2nd line) Median OS* (mo) Therapeutic Regimens for the Treatment of MCRC 24 ~15-20 18 ~12-14 ~11-12 12 6 ~15-17 ~4-6 0 1960s *Both first- and second-line exposure to therapy. MCRC = metastatic CRC; OS = overall survival. Avastin® (bevacizumab) PI. December 2004. Camptosar® (irinotecan) PI. July 2005. Eloxatin™ (oxaliplatin) PI. April 2005. Erbitux™ (cetuximab) PI. June 2004. Xeloda® (capecitabine) PI. April 2003. Vectibix (panitumumab) PI. 2006. 1980s 1990s 2000s 5-FU 5-FU biomodulation Irinotecan Oxaliplatin Cetuximab Bevacizumab Panitumumab Colorectal cancer: Evolution of 1st line therapy for metastatic disease. 5-Fluorouracil; 5-FU 5-FU/LV Foundation. U.S. Europe bolus infusional Mayo monthly Roswell Park weekly de Gramont bi-weekly First line: de Gramont vs Mayo 5-FU/LV 5FU: 425mgs/m2 x5d, q 4wks LV: 20 mgs/m2 x5d, q 4wks 500 pts randomized 40- p=0.0004 5FU: 400 mgs/m2(bolus) d1,2 q 2wks LV: 200 mgs/m2 d1,2 q 2wks 5FU: 600 mgs/m2(22hrs) d1,2 q 2wks 8- 33% 20- p=0.0012 27.6 15- 14.1 22 4- p=0.067 13- 13.1 14% RR (%) PFS (wks) OS (mos) de Gramont et.al. JCO 15:808 (97). Chemical Structure of Platinum Analogues NH3 Pt Diaminocyclohexane (DACH) carrier ligand NH NH3 O 2 C Cl CISPLATIN OXALATE hydrolysable ligand Pt NH2 Cl O C O NH3 O C O C Pt trans-l-diaminocyclohexane oxalatoplatinum OXALIPLATIN, Eloxatin® O NH3 O •Active in NCI CRC cell lines •DNA adducts, cross-links •Hold if CrCL<20cc/min CARBOPLATIN First line: FOLFOX vs infusional LV/5-FU Oxaliplatin 85 mg/m2 d1, q 2wks LV: 200 mg/m2; 5FU 400mg/m2 IVB 5FU 600 mg/m2 (22 hr CIV);d1,2q2wk 420 pts randomized 5FU: 400 mgs/m2(bolus) d1,2 q 2wks LV: 200 mgs/m2 d1,2 q 2wks 5FU: 600 mgs/m2(22hrs) d1,2 q 2wks 50- 51% p<0.0001 10- 9.0 p=0.001 18- 6.2 20- 5- 14- # p=0.12 16.2 14.7 22% RR (%) * PFS (mos) *=primary endpoint # OS (mos) deGramont et.al. JCO 18:2938 (2000). Irinotecan (CPT-11, Camptosar®) CH3 CH2 N O N N C O O N Irinotecan hydrochloride O HO O CH2CH3 •Topoisomerase I inhibitor an enzyme that relieves torsional strain in DNA •Hepatic metabolism. Majority excreted in bile requiring dose adjustment •Converted by the carboxylesterase enzyme to the SN-38 metabolite, which is 1000-fold more potent than the parent drug First line: FOLFIRI vs infusional LV/5-FU CPT-11 180 mg/m2+ deGramont q 2wks or CPT-11 80 mg/m2+HDLVB/5FU24CI qwk 385 pts randomized deGramont q 2wks or 500 LVB/5FU 2600mg/m2 24 CIV qwk 40- p<0.005 8- 35% 20- p=0.001 6.7 18- 17.4 4.4 4- p=0.031 14- 14.1 22% RR (%) PFS (mos) OS (mos) Douillard et.al. Lancet 355:1041 (2000). 5-FU/LV Foundation. U.S. Europe bolus infusional FOLFIRI Mayo monthly Roswell Park weekly FOLFOX de Gramont bi-weekly 5-FU/LV Foundation. U.S. Europe bolus infusional IFL Mayo monthly Roswell Park weekly FOLFIRI FOLFOX de Gramont bi-weekly First line: IFL vs Mayo 5-FU/LV 5FU: 425mgs/m2 x5d, q 4wks LV: 20 mgs/m2 x5d, q 4wks 457 pts randomized 40- 20- 39% p<0.001 21% RR (%) CPT11: 125 mgs/m2 LV: 20 mgs/m2 5FU: 500 mgs/m2; q wk 4/6 8- 4- 7.0 p=0.004 15- 14.8 p=0.04 4.3 13- PFS (mos) • 3rd arm: CPT-11 alone arm=LV/5FU 12.6 OS (mos) Saltz et.al. NEJM 343:905 (2000). 5-FU/LV Foundation. U.S. Europe bolus infusional ??? FOLFIRI FOLFOX IFL Mayo monthly Roswell Park weekly de Gramont bi-weekly First-Line Oxaliplatin in the US: N9741 Metastatic Disease Mayo trash R A N D O M I Z A T I O N N=264 Bolus Saltz Regimen* IFL CPT-11: 125 mg/m2/wk x 4 wks, q 6 wks 5FU: 500 mg/m2/wk x 4 wks, q 6 wks LV: 20 mg/m2/wk x 4 wks, q 6 wks Infusional FOLFOX-4 Regimen† N=267 Oxaliplatin: 85 mg/m2 d1 q 2 wks 5-FU: 400 IV/600 CI mg/m2 d1, 2 q 2 wks LV: 200 mg/m2 d1, 2 q 2 wks Wasserman Regimen‡ N=264 FOLFOX4 IROX CPT-11: 200 mg/m2 d1 q 3 wks Oxaliplatin: 85 mg/m2 d1 q 3 wks *Saltz et al. J Clin Oncol 14:2959, 1996. †André et al. J Clin Oncol 17:3560, 1999. ‡Wasserman et al. J Clin Oncol 17:1751, 1999. Goldberg J Clin Oncol 22:23, 2004 Europe vs USA? U.S. Euro Feb 2004: FOLFOX FDA approved as first-line chemotherapy in U.S. N9741: Efficacy IFL N=264 MS (mo) TTP (mo) 14.8 6.9 FOLFOX4 N=267 IROX N=264 CPT-11: 200 mg/m2 d1 q 3 wks Oxaliplatin: 85 mg/m2 d1 q 3 wks 19.5 17.4 (p=0.001) (p=0.04) 8.7 6.5 (p=0.0014) ORR (%) 31 45 35 (p=0.002) FOLFOX: Second-line approval in US 8/02; First line approval 2/04 Goldberg J Clin Oncol 22:23, 2004 FOLFIRI versus FOLFOX progression FOLFIRI FOLFOX6 progression FOLFIRI progression N=113 R N=113 progression FOLFOX6 Regimens: oxaliplatin* (100 mg/m2) or irinotecan† (180 mg/m2) IV + LV 200 mg/m2 over 2 hours d 1, 5-FU 2,400-3,000 mg/m2 over 46 hours Primary end point: TTP Secondary end points: ORR, safety Tournigand et al. J Clin Oncol. 2004 FOLFIRI verses FOLFOX6 Results Arm A FOLFIRI FOLFOX N=109 N=81 ORR % 56* 15† Arm B FOLFOX FOLFIRI N=111 P N=69 Value 4† .68 54* Median TTP (mo) 14.4 11.5 .65 Median Overall Survival (mo 20.4 21.5 .9 Tournigand C et al. JCO 2004 (Similar results: Colucci G, et al. J Clin Oncol. 2005;23:4866-4875). Molecular re-classification of metastatic colorectal cancer N I. ? II. . DIA HRN ? Molecular re-classification of metastatic colorectal cancer N I. II. Preliminary data: gene signature predicts diffuse metastatic disease . DIA HRN Incorporation of Targeted Agents in the Standard Management of Metastatic Colorectal Cancer Agents Targeting the VEGF Pathway Anti-VEGF antibodies VEGF Soluble VEGF receptors Anti-VEGFR antibodies P P P P VEGFR-1 Ribozymes P P P P VEGFR-2 Small-molecule VEGFR inhibitors (TKIs) Endothelial cell –VEGF stimulates new blood vessel formation in normal tissues and tumors –VEGF blockade normalizes tumor vasculature and improves drug delivery. Phase III Trial of Avastin + IFL as First-Line Therapy for MCRC (AVF2107g): OS 100 Median survival: 15.6 mo (w/o Avastin) vs 20.3 mo (w/Avastin) HR=0.66, P<0.001 OS (%) 80 Placebo + IFL (n=411) Avastin + IFL (n=402) 60 1-year survival: 74% vs 63% 40 20 2-year survival: 45% vs 30% 0 0 6 12 18 Months Error bars represent 95% CIs. Avastin® (bevacizumab) PI. December 2004. Data on file (SR2), Genentech, Inc. 2005. Hurwitz et al. N Engl J Med. 2004;350:2335. 24 30 EGFR as a Therapeutic Target The EGF Receptor: (HER1 or c-Erb-1) EGFR a member of a subfamily of type I receptor tyrosine kinases (including HER2, HER3 and HER4) EGF TGF- HER2/3/4 EGFR EGFR EGFR EGF = epidermal growth factor; TGF- = transforming growth factor-alpha; EGFR = epidermal growth factor receptor. Properties of ERBITUX (Cetuximab) IgG1 MAb (chimerized) Binds specifically to EGFR and its heterodimers Binds to EGFR with high affinity (Kd = 2.0 x 10–10 M): 1 log higher than the natural ligand Following the recommended dose regimen (400 mg/m2 initial dose/250 mg/m2 weekly dose), the mean half-life was 114 hours (range 75-188 hours) MAb = monoclonal antibody; EGFR = epidermal growth factor receptor. ERBITUX Package Insert, June 2004; Data on file. ImClone Systems Incorporated and Bristol-Myers Squibb Company; 2004. Please see Important Safety Information including WARNING regarding infusion reactions on slides 39 to 52. Cetuximab activity in irinotecan refractory mCRC (Bond Trial): Objective Response Rates All Patients Irinotecan-Refractory and Oxaliplatin Failure Patients Cunningham D, et al. N Engl J Med. 2004;351:337-345. Panitumamab +BSC vs BSC ORR 8% 0% • N=463 • Patients third-line mCRC NCIC-CO.17 Cetuximab+BSC N=287 mCRC failing (<6 months) oxaliplatin and CPT11 regimens with 5FU. No prior NOTE: No crossover allowed anti-EGFR Rx. Bev permitted. Primary endpoint=OS Median duration of f/u = 14.6 months BSC N=285 Jonker et. al, NEJM 357:2040-08 (2007) Jonker et. al, NEJM 357:2040-08 (2007) Primary endpoint OS Cetuximab plus bsc 6.1 mo 1 yr 21% p = 0.005 HR = .77 Bsc alone 4.6 mo 16% 7% cross-over from bsc-->cet Post trial rx: 27.5% (cet) verses 23.2% (bsc) What about in a “real world” setting? EPIC Sobrero et.al. AACR April 2007 CPT-11 *OS PFS RR 10.7 mo 3.98 mo 16.4% + cetuximab mCRC failing Oxaliplatin+/-bev 50% crossover (p = ns) (p < 0.0001) N=1298 * Primary Endpoint = OS Irinotecan: 350 mg/m2 q3weeks Cetux: 400mg/m2 load then 250mg/m2 CPT-11 9.99 mo 2.56 mo 4.1% What about upfront? CRYSTAL Primary Endpoint = PFS PFS FOLFIRI + Cetuximab 8.9 mo RR 46.9% (34% 1yr) Untreated mCRC p=0.036 p=0.005 8.0 mo 38.7% N=1217 FOLFIRI (23% 1yr) Van Cutsem E, et al. ASCO 2007. Abstract 4000. Metastatic colorectal cancer.... ? CALGB/SWOG80405 Trial: First Line Met CRC. Study PIs: Charles Blanke, M.D./Alan Venook, M.D. FOLFOX FOLFIRI Randomize “Dealers Choice” Bev C225 Bev/C225 Target accrual 2300 PACCE: Panitumumab Advanced CRC Evaluation Study Primary endpoint=PFS N >1000 • First-line mCRC • No CNS metastases • No CV risk factors R A N D O M I Z E Panitumumab + oxaliplatin- or irinotecan-based chemotherapy + bevacizumab (q2 wk) Oxaliplatin- or irinotecan-based chemotherapy + bevacizumab (q2 wk) Interim (12-week) response-rate analysis first 500 patients: response rates similar between treatment groups. US NIH Clinical Trials Database (www.clinicaltrials.gov); accessed 8/3/06 Cairo 2 Punt et. al ASCO 2008 Capox + bev + cetux PFS tox 9.6 mo 82% p=0.018 p=0.0013 N=368 mCRC Capox + bev N=368 10.7 mo 72% Cairo 2 Punt et. al ASCO 2008 PFS PFS Capox + bev + cetux PFS WT-KRAS MT-KRAS 9.6 mo 10.5 mo 8.6 mo N=368 p=0.018 mCRC Capox + bev N=368 p=ns p<0.05 10.7 mo 10.5 mo 12.5 mo CRYSTAL Van Cutsem E, et al. ASCO 2008. PFS PFS PFS WT-KRAS MT-KRAS FOLFIRI + Cetuximab Untreated mCRC N=1217 FOLFIRI 10Endpoint = PFS 8.9 mo 9.9 mo (34% 1yr) (43% 1yr) p=0.036 HR=0.85 p=0.017 HR=0.68 8.0 mo 8.6 mo (23% 1yr) (25% 1yr) p=0.75 HR=1/07 No change in PFS or RR in FOLFIRI w/o cetuximab Suggests KRAS a predictive marker (vs prognostic) OPUS trial: randomized phase II FOLFOX +/- cetuximab similar results that only WT-KRAS benefits from cetuximab (Bokemeyer et. al. ASCO 2008) Metastatic colorectal cancer.... ?????? The EGF Receptor: (HER1 or c-Erb-1) EGFR a member of a subfamily of type I receptor tyrosine kinases (including HER2, HER3 and HER4) EGF TGF- HER2/3/4 EGFR EGFR EGFR EGF = epidermal growth factor; TGF- = transforming growth factor-alpha; EGFR = epidermal growth factor receptor. CALGB/SWOG80405 Trial: First Line Met CRC. Study PIs: Charles Blanke, M.D./Alan Venook, M.D. Bev FOLFOX FOLFIRI Randomize “Dealers Choice” ONLY WT KRAS C225 Bev/C225 Target accrual 2300 ADJUVANT THERAPY TNM Stage I I IIA IIB IIIA IIIB IIIC IV TNM Class T1N0M0 T2N0M0 T3N0M0 T4N0M0 T1-2N1M0 T3-4N1M0 TanyN2M0 TanyNanyM1 MAC A B1 B2 ? B3 ? C1 C2/C3 C1/C2/C3 D N2 denotes 4 or more regional lymph nodes (Need to sample at least 12 nodes). (Modified Astler-Coller and New TNM Staging) COLORECTAL CANCER UPDATE Surgical Management Zollinger, Atlas of Surgical Operations, 1993 COLORECTAL CANCER UPDATE Surgical Management COLORECTAL CANCER UPDATE Surgical Management Total Mesorectal Excision • nerve preservation • sharp dissection • complete hemostasis • intact mesorectal envelope. Revised, node-positive TNM classification for Stage III CRC (n=50,042) IIIA: T1/2, N1 Observed 5-year survival (%) 70 60 IIIB: T3/4, N1 IIIC: Any T, N2 p<0.0001 59.8% 50 42.0% 40 27.3% 30 20 10 0 IIIA IIIB Node-positive subgroups IIIC Greene et al (2002) Adjuvant Therapy for Stage II Colon Cancer: • ASCO Guidelines : 8/15/04 JCO – “Pts with high risk features..might be considered candidates for adjuvant therapy..” BUT, – “Direct evidence does not support adjuvant chemotherapy..even for high risk stage II..” • http://www.mayoclinic.com/calcs/colon/index-ccacalc.cfm • Estimate that Stage II trials will need >8000 patients to provide direct evidence for benefit. • Need: better risk stratification to identify patient subgroups that will benefit (e.g. do-able trials). Adjuvant Therapy for Stage II Colon Cancer • Indirect evidence makes it reasonable to offer for patients with high risk features who are willing to accept toxicity for theoretical benefit. – High risk features: • • • • • < 12 LN sampled Obstruction at presentation Perforation at tumor site High grade tumor LVI • Should encourage participation in randomized trials. MOSAIC: Treatment arms NEJM 350(23):2343, 2004 FOLFOX4 (n=1123) Resected Stage II/III CRC N=2246 40% Stage II R LV5FU2 60% Stage III (n=1123) Endpoints Primary: – 3-yr Disease Free Survival (DFS): a surrogate for 5-yr Survival Secondary: – Safety – Overall Survival (OS) MOSAIC Stage III 4-year DFS 1.0 FOLFOX4 (n=672) LV5FU2 (n=675) Probability 0.9 70% 61% p=0.002 0.8 0.7 0.6 0.5 0 10 20 30 DFS (months) 40 50 24% risk reduction for stage III patients in the FOLFOX4 arm Improved 6 year overall survival for Stage III (hazard ratio of 0.80, confidence interval [0.65, 0.97], p=0.023). Phase III MOSAIC Trial (ITT): Clinical Efficacy Disease-free survival (DFS) FOLFOX LV/5FU2 Overall 76% 69% p=0.0008 risk reduction 24% Stage III 70% 61% p=0.002 Stage II 86.6% 83.9% p-value not significant de Gramont A, et al. Proc Am Soc Clin Oncol. 2003 Abstr 1015; Andre et.al NEJM 6/04 Irinotecan Negative in Adjuvant Setting Clinical Trial Patients Outcome CALGB C89803 Stage III (IFL vs FL) (n=1264) More neutropenia, death PETACC-3 More neutropenia, diarrhea (FOLFIRI vs deGramont) Stage II/III (n=3278) High-risk Stage III (LV5FU2+/-CPT11 (n=400) ACCORD02 Saltz et.al ASCO Ab 3500 (2004) Van Cutsem et.al ASCO Ab 8 (2005) Ychou et.al. Ab 3502 ASCO (2005) More neutropenia, nausea Molecular re-classification of metastatic colorectal cancer N I. ? II. . DIA HRN ? EORTC 40983 3 yr PFS FOLFOX x6 All pts Eligilble pts Surgery FOLFOX x6 Potentially resectable 35.4% 36.2% P=0.058 P=0.041 28.1% 28.1% N=182 mCRC No extrahepatic disease ≤4 lesions No prior Rx Primary endpoint – 40% increase in PFS (NOT designed to validate Surgery Pre vs post-op chemo) N=182 Nordlinger, ASCO 2007 EORTC 40983 • More postoperative complications in FOLFOX group 25% vs. 16% (p=0.04). (e.g. liver failure, biliary fistula). • No difference in postoperative mortality Nordlinger, ASCO 2007 Adjuvant colorectal cancer 1. FOLFOX for stage III (MOSAIC). -NSABPC07 (bolus 5FU+/- ox) confirms MOSAIC. FLOX with unfavorable toxicity profile? 2. No direct evidence for stage II pts. But, indirect evidence makes reasonable to discuss if high risk features • • • • • < 12 LN sampled Obstruction at presentation Perforation at tumor site High grade tumor LVI Some ongoing adjuvant trials Stage II CRC: E5202 Intergroup Trial MSS LOH 18q FOLFOX R FOLFOX + bevacizumab Stage II patients MSI+ normal 18q No therapy Accrual goal: 3,125 NSABP C-08: mFOLFOX6 + Bevacizumab • Patients with resected stage II/III colon cancer • Target enrollment, 2632 R A N D O M I Z E mFOLFOX6 + Bevacizumab q14 d for 12 courses Bevacizumab q14 d for 6 mo (in absence of PD) mFOLFOX6 q14 d for 12 courses Trial opened Sept 2004. ASCO 2008 Safety Update: increased grade III toxicity, p=0.006 US NIH Clinical Trials Database (www.clinicaltrials.gov); accessed 8/3/06. AVANT Trial: FOLFOX vs FOLFOX/BEV vs XELOX/BEV ????????????? • Patients with stage II/III colon cancer • Target enrollment, 3450 R A N D O M I Z E FOLFOX4 FOLFOX4 + Bevacizumab XELOX + Bevacizumab US NIH Clinical Trials Database (www.clinicaltrials.gov); accessed 8/21/06. N0147: Adjuvant Cetuximab For WT-KRAS ONLY!!!!!!!! • Patients with resected stage III colon cancer • Target enrollment, 2300 R A N D O M I Z E FOLFOX4 ± Cetuximab (FOLFOX then FOLFIRI) ± Cetuximab FOLFIRI ± Cetuximab US NIH Clinical Trials Database (www.clinicaltrials.gov); accessed 8/3/06. Reality Check...... • Cost: Shrag, NEJM, 7/2004 – Average patient with 1st line FOLFOX+ bevacizumab (8 months) followed by CPT-11 + cetuximab (4 months)= $161,000 USD X 50,000 Stage IV cases/year – > $5 BILLION USD /year (JUST for stage IV..if used for adjuvant..> $20 BILLION USD/year!!!! – How do we save $$$? • Lower cost • Limit therapy (per group) • Individualize treatments to those most likely to benefit (per patient) • Limit duration of therapy Colon Cancer: Multi-step Model of Carcinogenesis = biologic heterogeneity • • • • Cancers arise in polyps Develop over years Multiple molecular events Activation of oncogenes and loss of tumor suppressor genes Beart R. Clinical Oncology. Abeloff M, et al. Ed. 1998 Churchill Livingstone The Genomic Landscapes of Human Breast and CRC Cancers Wood et al. Science 2007;318:1108-13. Human cancer is caused by the accumulation of mutations in oncogenes and tumor suppressor genes. To catalog the genetic changes that occur during tumorigenesis, we isolated DNA from 11 breast and 11 colorectal tumors and determined the sequences of the genes in the Reference Sequence database in these samples. Based on analysis of exons representing 20,857 transcripts from 18,191 genes, we conclude that the genomic landscapes of breast and colorectal cancers are composed of a handful of commonly mutated gene "mountains" and a much larger number of gene "hills" that are mutated at low frequency. We describe statistical and bioinformatic tools that may help identify mutations with a role in tumorigenesis. These results have implications for understanding the nature and heterogeneity of human cancers and for using personal genomics for tumor diagnosis and therapy. A New Paradigm for Colorectal Cancer Clinical Trials Current A All patients receive standard treatment (A) patients B Clinical trials survival benefit from A Future A B patients Molecular analysis of tumor C D Choice of treatment dependent upon molecular profile of tumor and patient genotype Stay tuned for upcoming exciting info...........