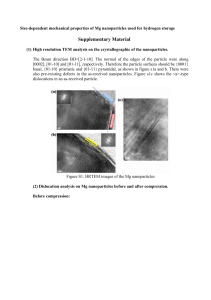
Lecture3.0 Defects and Solid Mechanics
advertisement

Lecture 3.0
Structural Defects
Mechanical Properties of Solids
Defects in Crystal Structure
Vacancy, Interstitial, Impurity
Schottky Defect
Frenkel Defect
Dislocations – edge dislocation, line,
screw
Grain Boundary
Substitutional Impurities
Interstitial Impurities
Self Interstitial
Vacancy
Xv~ exp(-Hv/kBT)
Vacancy Equilibrium
Xv~ exp(-Hv/kBT)
Defect Equilibrium
Sc= kBln gc(E)
Sb= kBln Wb
Ss= kBln Ws
Entropy
dFc = dE-TdSc-TdSs, the change in free energy
dFc ~ 6 nearest neighbour bond energies (since break on average 1/2 the bonds in the
surface)
Wb=(N+n)!/(N!n!) ~(N+n+1)/(n+1) ~(N+n)/n (If one vacancy added)
dSb=kBln((N+n)/n)
For large crystals dSs<<dSb
\
\n ~ N exp –dFc/kBT
Ionic Crystals
Shottky Defect
Frenkel Defect
Edge Dislocation
Grain Boundaries
Mechanical Properties of Solids
Elastic deformation
– reversible
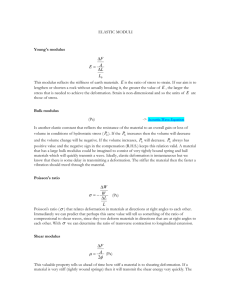
• Young’s Modulus
• Shear Modulus
• Bulk Modulus
Plastic Deformation
– irreversible
• change in shape of grains
Rupture/Fracture
Modulii
Shear
Young’s
Bulk
Mechanical Properties
Stress, xx= Fxx/A
Shear Stress, xy=
Fxy/A
Compression
Volume Strain = V/Vo
Brittle Fracture
Yield Stress
– yield ~Y/10
– yield~G/6 (theory-all
atoms to move together)
Strain, =x/xo
Shear Strain, =y/xo
– stress leads to crack
– stress concentration at crack
tip =2(l/r)
– Vcrack= Vsound
Effect of Structure on
Mechanical Properties
Elasticity
Plastic Deformation
Fracture
Stress
Plastic
Deformation
Fracture
Strain
Elastic Deformation
Pulling on a
wire decreases
its diameter
– Y(or E)= (F/A)/(l/lo)
– l/lo= -l/Ro
Poisson’s
Ratio, 0.5 (liquid
case=0.5)
Young’s Modulus
Shear Modulus
– G=/= Y/(2(1+))
Bulk Modulus
• K=-P/(V/Vo)
• K=Y/(3(1-2))
Microscopic Elastic Deformation
Repulsion
FC
Force
Interatomic Forces
FT =Tensile Force
FC=Compressive
Force
Note F=d(Energy)/dr
ao
r
0
FT
Attraction
Plastic Deformation
Single Crystal
– by slip on slip
planes
A cos
cos cos
a / cos
Yielding
o
yield
(cos cos ) max
o yield shear stress G / 30
Shear Stress
Deformation of Whiskers
Without Defects
Rupture
With Defects
generated by high stress
Poly Crystalline Copper
Dislocation Motion
due to Shear
Slip Systems in Metals
Crystal
Structure
Slip Planes
Slip
Directions
fcc
bcc
{111}
{110}
{211}
{321}
{0001}
{10-10}
{10-11}
<1-10>
<-111>
<-111>
<-111>
<11-20>
<11-20>
<11-20>
hcp
Number of
Slip
Systems
12
12
12
24
3
3
6
Examples
Al, Cu, Ni
Fe,Ta,W
Be, Mg,
Zn,Ti, Zr, Re
Plastic Deformation
Ao
Poly Crystals
– by grain
boundaries
– by slip on slip
planes
yield o k d
d grain size
Ai li Ao lo
– Engineering Stress, Ao
– True Stress, Ai
Ai
Movement at Edge Dislocation
Slip Plane is the plane on which the dislocation glides
Slip plane is defined by BV and I
Plastic Deformation
-Polycrystalline sample
Many slip planes
– large amount of
slip (elongation)
Strain hardening
– Increased difficulty of
dislocation motion due
to dislocation density
– Shear Stress to
Maintain plastic flow,
=o+Gb
• dislocation density,
Strain
Hardening
Strain Hardening/Work Hardening
Dislocation
Movement forms
dislocation loops
– New dislocations
created by
dislocation
movement
Critical shear
stress that will
activate a
dislocation source
c~2Gb/l
– G=Shear Modulus
– b=Burgers Vector
– l=length of
dislocation
segment
Depends on Grain Size
Burger’s VectorDislocations are characterised by their Burger's vectors. These
represent the 'failure closure' in a Burger's circuit in imperfect (top)
and perfect (bottom) crystal.
BV Perpendicular to Dislocation
BV parallel to Dislocation
Solution Hardening (Alloying)
Solid Solutions
• Solute atoms segregate to dislocations =
reduces dislocation mobility
• higher required to move dislocation
– Solute Properties
• larger cation size=large lattice strain
• large effective elastic modulus, Y
Multi-phase alloys - Volume fraction rule
Precipitation Hardening
Fine dispersion of heterogeneity
• impede dislocation motion
– c~2Gb/
• is the distance between particles
– Particle Properties
• very small and well dispersed
• Hard particles/ soft metal matrix
Methods to Produce
– Oxidation of a metal
– Add Fibers - Fiber Composites
Cracking vs Plastic Deformation
Brittle
• Poor dislocation motion
• stress needed to initiate
a crack is low
• good dislocation motion
• stress needed to initiate
slip is low
– Ionic Solids
– Metals
• disrupt charges
• electrons free to move
– Covalent Solids
• disrupt bonds
– Amorphous solids
• no dislocations
Ductile
Depends on T and P
– ductile at high T (and P)