The History of the Atom
advertisement

The History of the Atom
A Journey in the
realm of the unseen
Introduction
What does an atom look like? It is so small
that it cannot be seen. Yet we know there are
particles like protons, neutrons and electrons
that make up the atom.
How did scientists discover these subatomic
particles? This presentation will take you
through the scientists who contributed to the
discovery of the make-up of the atom.
The early Greeks defined all matter
As being rooted into the Four Elements.
Earth – all things that are dense
solids
Water – all things that are wet.
Wind – all things that float above.
Fire – Special stuff that have
both earth and wind in them.
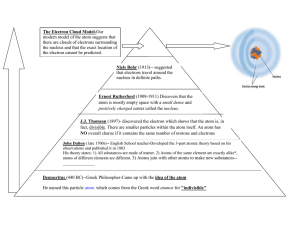
Democritus
Proposed that all matter is
composed of small bits of
matter too small to be
seen. These atoms
CANNOT be further split
into smaller portions.
There is a void, which is
empty space between the
small bits.
He called the bits of
matter “ATOMOS” Greek
for indivisible.
Greek philosopher
Democritus (460-371 B.C.)
Aristotle (384 BC – 322 BC)
Aristotle
Greek philosopher
Opposed Democritus’
beliefs
Believed that all matter
was continuous.
Antoine Lavoisier
Father of Modern Chemistry
Law of Conservation of Mass
In a chemical reaction, the total mass of the
reactants will equal the total mass of the
products
Antoine Laurent Lavoisier,
(1743-1794), French chemist
Joseph Proust
Law of Definite Composition (1799)
A compound always contains
the same elements in definite
proportions
Joseph Proust (1754-1826)
Example: pure sugar is
composed of: 42.1%
carbon, 51.4% oxygen,
& 6.5% hydrogen by
mass regardless of
where you find it.
Law of Definite Proportions
Regardless of how its combined, lead (IV) sulfide will always have
the same composition.
John Dalton
Developed the Law of
Multiple Proportions
First to recognize that
atoms could explain the
laws of: conservation of
mass, definite
composition and multiple
proportions
Proposed the Atomic
Theory in 1803
Five Part Atomic Theory
1.
2.
3.
All matter is composed of extremely small
particles called atoms.
Two or more atoms from the same element
are identical.
Atoms cannot be subdivided, created or
destroyed.
Five Part Atomic Theory
4. The Law of multiple proportions:
Atoms from different elements combine in
simple, whole-number ratios to form
chemical compounds.
5. In chemical reactions, atoms can be
combined, separated, or rearranged.
Dalton’s Model of the Atom
Dalton developed a model of the atom based
on his atomic theory. He felt the atom was an
extremely small, indivisible particle.
His model of the atom is called the Billiard
Ball Model.
Solid Sphere
JJ Thomson
Experimented with a CRT
(cathode ray tube)
A CRT is an evacuated glass
bulb containing two ends: the
cathode and the anode.
Cathode Ray Tube
An electrical current passes through the tube
from the cathode (negative end) to the anode
(positive end).
Thomson studied the cathode ray that traveled
from the cathode to the anode and noticed that
the cathode rays were the same regardless of
the element or metal used to make-up the
cathode.
Cathode Ray Tube
A magnet was applied to these rays and always
with the same results:
Negative end of magnet repelled cathode ray;
Positive end of magnet attracted cathode rays.
Thomson’s Conclusion
Cathode ray is made up of an extremely small
particles that are common to all matter.
The particle have a negative charge.
Thomson discovered the ELECTRONS.
Robert Millikan
Oil Drop Experiment
(1909)
Work contained excellent
precision
Determined the exact
charge and exact mass of
an electron
Oil Drop Experiment
How the Oil Drop Experiment
Worked
A fine mist of oil is sprayed into the chamber.
A few oil drops will fall through the hole in the
positively charged plate at the top.
As the oil drops fall due to gravity, they acquire
extra electrons which are dislodged from gases in
the air by X rays.
As the charged oil drops descend, the electrically
charged plates are turned on.
How the Oil Drop Experiment
Worked
The oil drops now have two forces acting on
them. Gravity and electrical charge.
Using the microscope to observe the oil drops,
Millikan could determine the charge needed to
suspend the drops in mid-air.
Millikan calculated the:
exact mass (9.109 x 10-28 grams) and
charge (-1.6 x 10-19 coulombs) of an electron.
Results of CRT and Oil Drop
Experiment
1.
2.
3.
4.
Proved that atoms are divisible.
Atoms are electrically neutral
therefore they must have a
positive charge equal to the
negative charge.
Since electrons have such a small mass,
atoms must have additional particles to
account for most of their mass.
The Plum pudding model was created &
confirmed.
Ernest Rutherford
Thought that the atom was all empty space.
Used the Gold Foil Experiment to test his
hypothesis. (1908 and 1909)
Gold Foil Experiment
Almost all of the particles pass through
with a slight deflection BUT some
particles came back.
1 in 8,000 particles ricocheted back to the
source
Gold Foil Experiment
Rutherford said it was “as if you
had fired a 15-inch (artillery) shell at
a piece of tissue paper and it came
back and hit you.”
Why did this happen?
Rutherford reasoned that the fast-moving particles
must be repelled by some powerful force within
the atom. Also, whatever caused this repulsion
must occupy a very small amount of space since
only a very few particles ran into it.
The Nucleus
So how small is the nucleus?
How large is an atom’s volume compared to its
nucleus?
Think of a football field and place a dime in the
center of the 50 yard line.
Rutherford’s Atom
The dime represents the nucleus of the atom
while the outer edge of the football field would
represent the outer edge of the atom.
Rutherford concluded that the atom
is mostly empty space.
Rutherford’s model of the
atom is the nuclear model.
A Puzzle
If an atom has a positive center and the
negative electrons are on the outside of the
atom, why don’t the electrons fall into the
center?
………………..Centrifugal force
inertia force due to e- traveling in circles.}
{an
Niels Bohr
Developed the Planetary
Model in 1913
Electrons move around
the nucleus like planets
move around the sun.
Bohr
Bohr suggested that electrons travel in a
specified path around the nucleus which he
called energy levels. These energy levels are
designated distances from the nucleus in which
electrons may be found.
The maximum number of
electrons found in an energy
level can be determined by
the formula 2n2, where
n = energy level.
Werner Heisenberg
1927 – Heisenberg
Uncertainty Principle
It is not possible to
know both the velocity
and the position of an
electron at the same
time.
Erwin ShrÖdinger
Austrian Physicist who
developed an e- formula.
His theory was able to
determine the most likely
AREA an e- is to be found.
These areas are called Orbitals
James Chadwick
Discovered the neutron in
1932
The neutron is a particle in
the nucleus that has about
the same mass as a proton,
but has no charge.
Modern Day Model
Two Main Parts
The Nucleus
Positively Charged
PROTONS
Neutral NEUTRONS
Held together by the
STRONG NUCLEAR
FORCE
The Electron Cloud
e- moving about the
nucleus in
3-D ORBITALS (s,p,d,f).
The e- ORBITALS are
positioned in ENERGY
LEVELS
Properties of Subatomic Particles
Particle
Electron
Charge
e- -1
Proton
p+
Neutron
n
+1
0
Mass Relative mass
#
(a.m.u.)
Actual mass
(grams)
0
0.0005486
9.109 x10
-28
1
1.007276
1.673x 10
-24
1
1.008665
1.675x 10
-24
Fuzzy Blob of Uncertainty
The Modern Model
Modern Day Model of the
ATOM
The modern day model is a collection of all
the contributions of the previous scientists,
from Dalton to Chadwick.
Today we would have to include quarks
which make-up the protons and neutrons in
the nucleus.
MURRAY GELL-MANN named the 6
Quarks after a line in the play “Finnegan’s
Wake.” A good bonus questions might be to
name these six flavors of quarks.
THE END
Are you ready for
the history of the
Atom quiz?