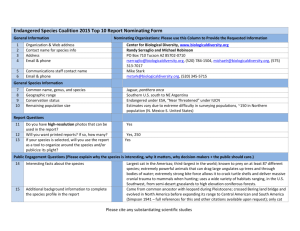
Status, conservation, and population genetics of the jaguar
advertisement

Status, Conservation, and Population Genetics of the Jaguar (Panthera onca) in Paraguay and the Dry Gran Chaco by Anthony J. Giordano, B.Sc. (Hons), M.Sc. A Dissertation In Wildlife, Aquatic, & Wildlands Science Submitted to the Graduate Faculty ofTexasTechUniversity in Partial Fulfillment of the Requirements for the Degree of DOCTOR OF PHILOSOPHY Approved Philip S. Gipson Co-Chair of Committee Mark Wallace Co-Chair of Committee Melanie Culver Nancy McIntyre Gad Perry Mark Sheridan Dean of the Graduate School May, 2015 Copyright 2015, Anthony J. Giordano Texas Tech University, Anthony J. Giordano, May 2015 ACKNOWLEDGMENTS Countless people and organizations have played at least some role in making this dissertation a reality. Foremost, my fondest memories are of my time with the late Dr. Warren B. Ballard. His faith in my potential, persistent encouragement and guidance, and our many mutually shared interests, were among the most important factors in my return to graduate school. His professional advice and personal friendship are missed every day, and I owe him more than I can express in words. Thanks to Dr. Philip S. Gipson, a long-time colleague, for stepping in as my co- major professor, and for his continued guidance and friendship. I am also grateful to Dr. Mark Wallace, my co-major professor, for his sustained efforts to ensure support for my program. I am equally grateful to Dr. Melanie Culver, for her hospitality and support at the University of Arizona, an essential part of a creative partnership that ultimately made this work possible. My sincerest thanks also to Dr. Nancy McIntyre for her friendship, guidance, and encouragement, and to Dr. Gad Perry for sharing his own experiencesin international conservation,as well asnumerous insights he has gained during this time. Significant fellowship and scholarship support for my work was provided by the Texas Tech Graduate School, the TTU Department of Natural Resources Management, the Sigma Xi Scientific Society, The Explorer’s Club, the Golden Key International Honor Society, the ARCS Foundation, the Houston Safari Club, the Wild Felid Research & Management Association, and the U.S. State Department’s Fulbright Program in cooperation with the Fulbright Foundation. I am also thankful for the support of Panthera’s Kaplan Graduate Award Program, and the influence and support of Dr. Alan Rabinowitz, particularly for the guiding role he played during a pivotal point early in my career. Additional grant support was provided for various aspects of this project by the Woodland Park Zoo’s Jaguar Conservation Fund;the Wildlife Conservation Society, particularly early on in this project;the Sedgwick County Zoo; S.P.E.C.I.E.S.;and the United States Fish & Wildlife Service’s International Programs Division. A considerable amount of logistical and in-kind support was provided by ii Texas Tech University, Anthony J. Giordano, May 2015 Guyra Paraguay, and my sincerest thanks to them for their continued support and partnership. I would also like to thank Fundacion Moises Bertoni, Red Paraguaya, the Scientific Society of Paraguay, and the University of Asuncion for the help they provided over the many twists and turns that project activities often took. Finally, I would like to thank the Secretariat of the Environment of Paraguay (SEAM) for the permission to do this project, and their continued support of my jaguar research program. I would like to thank Dr. Alberto Yanosky for his enthusiasm, guidance, and unwavering support during my dissertation research. My thanks to Silvino Gonzalez of the SEAM, Superintendent of Chaco Defensores National Park, for sharing with me an invaluable lifetime of knowledge about the Gran Chaco, and for the countless hours he contributed to my project. Thanks also to the entire Culver Conservation Genetics Laboratory at the University of Arizona for their support. Many individuals volunteered time to this project either in the field (Paraguay) or the laboratory (Arizona), too many to name them all. However, I’m especially thankful to Marianela Velilla, Nathalia Mujica, Silvia Saldivar, Fredy Ramirez, Sophia Amirsultan,Eldridge Wisely, Karla Vargas, and Polina Kamalova, without whom this project may not have been possible. I am also grateful to Ashwin Naidu and Robert Fitak for their assistance and insights during my work in the laboratory, and Peter Clark and Damian Rumiz for their support in providing graphics and other materials, as well as permission to reproduce them. Finally, I’m not sure where I’d be now without my family’s lifelong support and encouragement, and their constant faith in me. To them, I owe everything. iii Texas Tech University, Anthony J. Giordano, May 2015 TABLE OF CONTENTS ACKNOWLEDGMENTS .................................................................................. IIi LIST OF TABLES ........................................................................................... VIIi LIST OF FIGURES .......................................................................................... IXx I. INTRODUCTION ............................................................................................. 1 II. THE JAGUAR IN PARAGUAY: DISTRIBUTIONAL RECORDS AND THREATS TO A DECLINING TOP CARNIVORE ........................................ 5 ABSTRACT ...................................................................................................... 5 INTRODUCTION ............................................................................................ 6 METHODS ....................................................................................................... 8 JAGUAR RECORDS ..................................................................................... 10 CURRENT THREATS TO JAGUARS .......................................................... 13 Eastern Paraguay ....................................................................................... 13 Gran Chaco................................................................................................ 16 MANAGEMENT RECOMMENDATIONS .................................................. 19 REFERENCES................................................................................................ 21 III. ADAPTATION OF NONINVASIVE GENETIC TECHNIQUES FOR IDENTIFYING JAGUARS AND PUMAS IN THE GRAN CHACO: PRACTICAL CONSIDERATIONS AND IMPLICATIONS......................... 37 ABSTRACT .................................................................................................... 37 INTRODUCTION .......................................................................................... 38 STUDY AREA ............................................................................................... 40 MATERIALS & METHODS ......................................................................... 41 Scat Collection .......................................................................................... 41 Scat Storage and Processing ..................................................................... 41 Species Identifications .............................................................................. 42 Sex Identification ...................................................................................... 43 Potential Factors Impacting Species Identification Success ..................... 44 RESULTS ....................................................................................................... 45 DISCUSSION ................................................................................................. 47 Efficacy of NGS Approach ....................................................................... 48 Jaguar and Puma Samples ......................................................................... 51 iv Texas Tech University, Anthony J. Giordano, May 2015 CONCLUSIONS ............................................................................................. 54 REFERENCES................................................................................................ 57 IV. ABUNDANCE & DENSITY OF THE JAGUAR (PANTHERA ONCA) IN THE PARAGUAYAN DRY CHACO: USE OF A NONINVASIVE GENETIC SAMPLING FRAMEWORK ............................................................................ 73 ABSTRACT .................................................................................................... 73 INTRODUCTION .......................................................................................... 75 STUDY AREA ............................................................................................... 77 METHODS ..................................................................................................... 78 Field Sampling .......................................................................................... 78 Genotyping & Error-Checking .................................................................. 78 Population Size & Density ........................................................................ 80 RESULTS ....................................................................................................... 80 Quality Control & Consensus Genotypes ................................................. 81 Abundance & Density ............................................................................... 81 DISCUSSION ................................................................................................. 82 Identifying Individuals in a NGS Framework ........................................... 82 Use of Scats in Closed Capture-Recapture Estimators ............................. 83 Jaguar Density in the Gran Chaco ............................................................. 84 REFERENCES................................................................................................ 90 V. JAGUAR (PANTHERA ONCA) POPULATION GENETICS ACROSS A DRY CHACO-PANTANAL LANDSCAPE: ASSESSING GENE FLOW BETWEEN HIGH PRIORITY JAGUAR CONSERVATION STRONGHOLDS ............................................................................................................................. 108 ABSTRACT .................................................................................................. 108 INTRODUCTION ........................................................................................ 109 METHODS ................................................................................................... 112 Sample Collection & Species Identification ........................................... 112 Genotyping .............................................................................................. 112 Genetic Diversity & Variation ................................................................ 114 Genetic Differentiation & Isolation By Distance .................................... 114 RESULTS ..................................................................................................... 116 Genetic Diversity & Variation ................................................................ 117 Population Differentiation & Structure ................................................... 118 DISCUSSION ............................................................................................... 121 v Texas Tech University, Anthony J. Giordano, May 2015 Genetic Diversity & Variation ................................................................ 121 Population Differentiation & Structure ................................................... 123 Conservation Implications ...................................................................... 127 REFERENCES.............................................................................................. 129 VI. SUMMARY ................................................................................................. 162 APPENDIX A. JAGUAR RECORDS FROM PARAGUAY ...................... 165 APPENDIX B. SAMPLE RECORDS USED IN POPULATION GENETICS ANALYSIS ........................................................................................................ 169 vi Texas Tech University, Anthony J. Giordano, May 2015 LIST OF TABLES 1.1 Summary of jaguar records across Paraguay’s ecoregions by evidence......................................................................................... 36 2.1. Species identification of scat samples by DNA isolation attempt ................. 70 2.2. Individual variation in species ID success as determined by laboratory performance in DNA Isolation from scat samples. ......................................................................................... 71 2.3. Success in sex identification of large felid scat samples by PCR amplification attempt .................................................................... 72 3.1. Jaguar scat samples collected (April – August 2009), % of samples successfully yielding multi-locus consensus genotypes, unique genotypes, and the Probability of Identity for Siblings for each CR-NGS event at DCNP. ................................ 105 3.2. Estimates of jaguar abundance using the TIRM in capwire for each of three CR-NGS events at DCNP. CI = confidence interval; Na= estimate of ‘easy-to-capture’ individuals; Nb= estimate of ‘difficult-to-capture’ individuals; α = CR parameter. ............................................................................. 106 3.3. Estimates of effective sampling area and jaguar density at DCNP for three CR sampling events. ..................................................... 107 4.1. Measures of genetic diversity and for 8 microsatellite loci for 40 individual jaguar scat samples from the Paraguayan dry Chaco (N = sample size, SR = allele size range (base pairs), AN = observed number of alleles, HO = Observed Heterozygosity, HE = Expected Heterozygosity, uHE = unbiased I = Shannon’s Information Content, PA = Private Alleles, and P(ID-SIB) = probability of identity for siblings) ....................................................................................... 142 4.2. Comparisons of overall measures of genetic diversity for individual jaguars originating from the Paraguayan Chaco and Brazilian Pantanal based on scat collections (AN = mean observed number of alleles per locus, AR = estimate of allelic richness, HO = observed heterozygosity, HE = expected heterozygosity, I = Shannon’s information content, PAO = observed number of private Alleles, PAE = estimated number of private alleles, and P(ID-SIB) = probability of identity across all loci) ............................................................................................. 143 4.3. Pairwise estimates of measures of population differentiation between jaguars in the Paraguayan Chaco (PC) and the Brazilian Pantanal (BP) [FST, GST, G’ST, Nei’s G’ST, vii Texas Tech University, Anthony J. Giordano, May 2015 &DEST & G’’ST] and the effective number of migrants (Nm) for each locus and the mean across all loci. ....................... 144 viii Texas Tech University, Anthony J. Giordano, May 2015 LIST OF FIGURES 1.1 Map depicting jaguar records in Paraguay with respect to the country’s ecoregions and protected areas. Legend: direct observations (dark circles), interview records and field sign (open circles with X’s), field sign alone (X’s), and interviews alone (open circles). Anecdotal (secondhand) evidence and reports with no additional information are indicated by an “a”, while “?” apply to broad areas where evidence of jaguars is completely lacking. ......................... 30 1.2. Map of Paraguay’s political departments. The Gran Chaco occupies Presidente Hayes, Boqueron, and Alto Paraguay; the dark shape represents the location of Chaco Defensores National Park to scale at 7,800 km2 in area. ............................................................................................... 32 1.3. Map of protected areas with approximate extent of ecological regions. This map depicts the Chaco-Pantanal transitional zones as “Chaco Humedo” and also notes the pockets of cerrado along the extreme northern border (i.e., ‘Chovereca’ region) with Bolivia in the dry Chaco and Pantanal. Excerpted and reproduced with permission of Peter T. Clark ( © 2014). .......................................................... 34 2.1. Map of Paraguay showing the location of Defenders of the Chaco National Park................................................................................. 68 3.1. Coarse boundary map for the general ecoregions of Paraguay, showing the location of Defenders of the Chaco National Park (black) in the northern dry Chaco. ....................... 101 3.2. The portions of roads bordering and transecting DCNP serving as regular jaguar scat sampling transects for all systematic sampling events. .......................................................................... 103 4.1. Map depicting the relative location of core sampling areas. The red polygon depicts where sampling occurred in Gran Chaco Jaguar Conservation Unit (left, Paraguay), while the green polygon depicts where samples were collected in and the Pantanal Jaguar Conservation Unit (right, Brazil). The closest samples from these areas were approximately 280 km apart........................................................ 140 4.2a. Mantel test of the linear geographical distance (x) and Nei’s genetic distance (y) for 50 individual jaguars originating from the Paraguayan Chaco and Brazilian Pantanal (p<0.001). .................................................................................... 145 ix Texas Tech University, Anthony J. Giordano, May 2015 4.2b. Mantel test of the log-transformed geographical distance (x) and Nei’s genetic distance (y) for 50 individual jaguars originating from the Paraguayan Chaco and Brazilian Pantanal (p<0.001). ..................................................................... 147 4.3. Regression coefficient estimates of geographical vs. genetic distance plotted over multiple expanding distance classes (km) of pairwise comparisons between jaguar samples. The graph demonstrates weak (r stabilizes at ~0.016) but significant spatial autocorrelation between genetic and geographical distance ............................................... 149 4.4. Mean population probability assignment (q) for 50 individual jaguars for 10 iterations of K = 2 where population origin was specified. The dark orange coloration represents the probability that an individual is assigned to the population originating from the Gran Chaco of Paraguay, while the green represents the probability an individual is assigned to the Brazilian Pantanal. The 10 individual jaguars originating from the Pantanal are all toward the right of the graph. ...................................................... 151 4.5. Plot of variation in ΔK, or the mean of the absolute value of the 2nd order of the likelihood distribution (|Mean(L”(K)|) over the standard deviation of the mean likelihood values (SD (L(K)), with successive values for K (K = 29); ΔK is maximized for K=2...................................................... 153 4.6. Mean population probability assignment (q) for 50 individual jaguars for 10 iterations of K = 2 where population origin wasn’t specified a priori. The dark orange coloration represents the probability that an individual is assigned to the population originating from the Gran Chaco of Paraguay, while the green represents the probability an individual is assigned to the Brazilian Pantanal. The 10 individual jaguars originating from the Pantanal are all toward the right of the graph. ............................ 155 4.7a, b. Maps of varying extent depicting the geographical location of jaguar scat samples collected from the Paraguayan Chaco (yellow pins, Alto Paraguay) and Brazilian Pantanal (green and red pins, Mato Grosso do Sul). Both maps show in detail the extensive deforestation and development that has occurred in both the Chaco uplands and Pantanal floodplains. ............................................... 157 x Texas Tech University, Anthony J. Giordano, May 2015 CHAPTER I INTRODUCTION The jaguar (Panthera onca) is the world’s third largest felid and the largest obligate terrestrial carnivore in Latin America. Since the early 1900’s, the jaguar’s range has declined considerably, and it is now believed to occupy less than half of its former distribution. While jaguars have increasingly been the subject of more intensive research and conservation efforts in recent years, most of these activities have arguably occurred across arelatively small proportion of the cat’s range, leaving large geographical regions where the jaguar’s status still remains poorly assessed. Among these latter regions are large portions of the Gran Chaco of southcentral South America. Although the Bolivian and Argentine Chaco have both been the subject of prior jaguar surveys, the Paraguayan Chaco, comprising approximately one-third of the extant Gran Chaco and occurring between these two regions, has been devoid of such efforts. This lack of data detracts from important ecoregional and transboundary conservation planning efforts, as the Paraguayan Chaco figures prominently as part of the Gran Chaco Jaguar Conservation Unit (JCU), which is part of a rangewide network of stronghold habitats important to long-term jaguar population persistence. To illustrate the conservation status of jaguars across the entire Gran Chaco region, better inform regional and transboundary jaguar conservation planning efforts and the development of an effective jaguar conservation strategy for the Gran Chaco, and provide greater context of the relationship between jaguars in the Gran Chaco JCU and those in the adjacent Pantanal JCU, scientific investigations of the jaguar populations in northern Paraguay are urgently needed. In the following chapters, I present an original investigation into the status, ecology, and population genetics of jaguars in Paraguay, with a strong emphasis on the northern dry Chaco of Paraguay. My first core chapter (Chapter II: The Jaguar in Paraguay: Distributional Records and Threats to a Declining Top Carnivore) is pre-formatted for submission to the journal, Studies in Neotropical Fauna and the Environment. Style with respect to references and citations, word limits, the location of figures and tables, and other 1 Texas Tech University, Anthony J. Giordano, May 2015 aspects of this chapter all follow author submission guidelines for this journal, and I frequently use “we” to refer to my intended co-authors, which are comprised of committee members, and laboratory and field collaborators. I also make reference to myself and some of my co-authors using initials. In this chapter, I present a synthesis of the distribution of jaguars across Paraguay, incorporating the details of both direct and indirect records from a variety of qualified sources. I make conclusions regarding the general status of the jaguar across several major ecoregions of Paraguay, as well as the threats jaguars face in these regions. I also discuss the implications of these threats, and make recommendations for potential mitigation management practices and policies that would improve the jaguar’s conservation status in the region. Chapter III (Adaptation of Noninvasive Genetic Techniques for Identifying Jaguars and Pumas in the Gran Chaco: Practical Considerations And Implications) is pre-formatted for submission to the journal Integrative Zoology, and style with respect to references and citations, word limits, the location of figures and tables, and other aspects of this chapter all follow author submission guidelines for this journal. Here as for the first chapter, I frequently use “we” to refer to my intended co-authors, which are comprised of committee members, and laboratory and field collaborators. In Chapter III, I test the efficacy of noninvasive genetic sampling technique for monitoring and differentiating jaguars and pumas in the Paraguayan Dry Chaco. I examine factors that could potentially impact and improve success in field and laboratory methods, and make recommendations regarding optimal approaches for collecting, storing, and processing felid scats. I also examine the demographic and species composition of scats collected in and adjacent to Paraguay’s largest inviolate protected area, Defensores del Chaco (“Defenders of the Chaco”) National Park, and discuss the implications of these results in the context of prior research and future sampling efforts. My fourth chapter (Chapter IV: Abundance & Density of the Jaguar (Panthera onca) in The Paraguayan Dry Chaco: Use of a Noninvasive Genetic Sampling Framework) is pre-formatted in accordance with the guidelines of the journal,Animal Ecology. Here as with prior chapters, style with respect to references and citations, 2 Texas Tech University, Anthony J. Giordano, May 2015 word limits, the location of figures and tables, and other aspects of this chapter all follow this journal’s guidelines, and I frequently use “we” to refer to my intended coauthors. I also refer to previous chapters as “Giordano et al., in prep” followed by the Chapter number (e.g., “Giordano et al., in prep; Chapter 3”) as a means to easily adapt these citations to accurately reflect the prior’s chapters status prior to the submission of this chapter. Chapter IV addresses the lack of quantitative information on jaguars in Paraguay by providing the first estimate of jaguar abundance for the country, as well as the first noninvasive genetic estimate of jaguar abundance for the Gran Chaco ecoregion. I calculated these estimates by sampling a large geographical region and using a likelihood-based capture-recapture approach robust to small population sizes and heterogeneity in capture probability, two factors I anticipated in sampling jaguars noninvasively. I then derive realistic estimates of jaguar density for the Park based on previously-collected home data, and discuss the implications of these estimates for the entire Gran Chaco in the context of prior research in Bolivia. Finally, I have formatted the fifth chapter (Chapter V: Jaguar (Panthera onca) Population Genetics Across a Dry Chaco-Pantanal Landscape: Assessing Gene Flow Between High Priority Jaguar Conservation Strongholds) for the open access journal, PLOS One. The unique numbered citation format here is in accordance with this journal’s requirements, as are word limits and the formatting for figures and tables. . As in Chapter IV, I also refer to previous chapters as “Giordano et al., in prep” followed by the Chapter number (e.g., “Giordano et al., in prep; Chapter 3”) as a means to easily adapt citations to reflect the prior’s chapters review or publication status prior to the submission of this chapter for peer review. Chapter V represents the first known transboundary investigation of jaguar ecology and population genetics. In this chapter, I examine the genetic diversity of jaguars in the Paraguayan Dry Chaco using noninvasive scat samples. By incorporating additional samples collected from the Brazilian Pantanal, I also investigate evidence for connectivity between jaguar populations in the two adjacent regions. Specifically, I developed multiple lines of evidence in interpreting tests of isolation-by-distance, differentiation, and population structure between both populations, and compare and contrast measures of genetic 3 Texas Tech University, Anthony J. Giordano, May 2015 diversity between individual jaguars in both regions. I then discuss and interpret my findings in the context rangewide jaguar conservation planning efforts, which emphasize the connectivity of viable jaguar habitat from northern Mexico to northern Argentina. 4 Texas Tech University, Anthony J. Giordano, May 2015 CHAPTER II THE JAGUAR IN PARAGUAY: DISTRIBUTIONAL RECORDS AND THREATS TO A DECLINING TOP CARNIVORE ABSTRACT The local distribution and occurrence of the jaguar (Panthera onca), the largest obligate carnivore in the Western Hemisphere, is poorly known across large parts of its range. Gaps in knowledge regarding the presence or absence of any species, and a lack of information on conservation status and specific threats in certain regions, can delay the development of conservation strategies and action plans, impede the establishment of protected areas and biological corridors, and delay transboundary or otherwise cooperative conservation activities. Here we describe what is currently known about the distribution and status of jaguars in Paraguay, including its frontier regions with Bolivia, Brazil, and Argentina. In most cases we emphasize the Paraguayan Chaco, but make reference to related threats throughout the Gran Chaco biome, as well the various ecoregions of eastern Paraguay. Occurrence records were compiled from a variety of sources, including biologists and conservation professionals, land managers, regional non-governmental conservation organizations, and Paraguay’s Secretariat of Environment (SEAM). We obtained additional records from field work conducted by our team, including direct observations, track observations, genetic confirmation of scats, and photos from camera-traps. We interpret the general status of the jaguar based on these records, and discuss them in the context of existing and emerging threats to jaguars in Paraguay and across the Chaco. As the drivers of threats to jaguars are complex and must be considered across large landscapes, their persistence or resolution has similar consequences for many species, making jaguars effective “umbrellas” for habitat conservation. We offer recommendations based on the local socioeconomic, political, and cultural contexts for the mitigation of these threats; this includes measures pertaining specifically to jaguars, as well as more generally to Chaco habitat. 5 Texas Tech University, Anthony J. Giordano, May 2015 INTRODUCTION The ‘Near Threatened’ jaguar (Panthera onca) (IUCN 2008) is the western hemisphere’s largest felid, and the apex predator of theentire Neotropical region. Today the jaguar is estimated to occupyno more than 46% of itshistoric distribution at the turn of the 20th century (Sanderson et al. 2002). Jaguars were also arguably among the last of the big cats to receive serious research attention. This may have been due largely to their comparatively solitary and cryptic nature (Furtado et al. 2008), and the difficulties of accessing and sampling their typical forest or flooded wetland habitats. Following the rapid emergence and widespread adoption of camera-trapping techniques by wildlife biologists in the 1990’s, estimates of jaguar population size using capture-recapture (CR) camera-trapping methods first applied to tigers (e.g., Karanth 1995; Karanth & Nichols 1998) were subsequently applied to jaguars across a number of habitats and geographical regions (e.g., Wallace et al. 2003; Maffei et al. 2004; Silver et al. 2004; Soisalo & Cavalcanti 2006; Silveira et al. 2009). As a partial consequence, jaguars have been the focus of both increasing research and conservation attention over the past three decades (Astete et al. 2008), including several synthesis and conservation planning workshops (e.g., Sanderson et al. 2002; Grigione et al. 2009). Depsite the greater sustained focus on jaguars in many regions of Latin America, it is relatively surprising that information regarding the status of jaguars, and the specific geographical threats they face, is lacking from broad parts of their range (Sanderson et al. 2002). One problematic consequence of this is an asymmetry in knowledge or data quality, and the resulting challenges of implementing effective jaguar conservation measures in those areas. An incomplete understanding of the jaguar’s statusmight precipitate biases in regional surveys or conservation efforts, while limited financial resources in the absence of basic information could make investments in jaguar conservation efforts elsewhere less probable or possible. Conversely, the reporting of informative occurrence records from regions where information is completely lacking may ultimately lead to anincrease in research, collaboration, and conservation emphasis in those areas. As an example, a recent 6 Texas Tech University, Anthony J. Giordano, May 2015 history of published accounts of jaguar occurrences across Mexicohave expanded the scope of and emphasis on work there (e.g., Navarro-Serment et al. 2005; MonroyVilchis et al. 2008;Rosas-Rosas& Lopez-Soto 2002; Villordo-Galvan et al. 2010). Surveys, record gathering, and more detailed investigations of jaguar ecology are presently occurring, or have recently occurred, across disparate parts Mexico, including the states of Sonora, Tamaulipas, Nuevo Leon, Sinaloa, Mexico, Michoacan, San Luis Potosi, Oaxaca, Puebla, Quintana Roo, Campeche, Chiapas, Nayarit, and Jalisco (Monroy-Vilchis et al. 2008, Villordo-Galvan et al. 2010, de la Torre & Medellin 2011, Briones-Salas et al. 2012, Charre-Medellin et al. 2013, Petracca et al. 2013;A. J. Giordano, pers. obs.; 2013 conversations with R. Carerra and R. Thompson). An equally pressing priority is the lack of specificity or context regarding the underlying threats driving regional jaguar declines. Although threats to jaguars are generally and categorically well understood across their range, the drivers can vary in great detail among countries and cultures, particularly at a fine scale. Few regional assessments of threats to jaguars exist, despite important broad-scale planning efforts to connect regional populations across the jaguar’s range (Sanderson et al. 2002; Rabinowitz &Zeller 2010). Yet the implications of these threats in the long-term, as well as the socioeconomic and ecological context in which their drivers occur,are particularly important to the development of effective mitigation strategies. For example, while actual and perceived human-jaguar conflict represents a general pervasive threat common to many jaguar populations (Rabinowitz 1995; Crawshaw 2003; Rabinowitz 2005; Silveira et al. 2008), the varying cultural, socio-economic, and ecological contexts in which these threats across the jaguar’s range often demand related but context-specific solutions. Other threats, such as an international U.S.Mexico border wall (McCain & Childs 2008), or the expansion of oil palm agribusiness (Euwe et al. 2013; Panthera Colombia, unpub. data), may be unique to certain geographical regions. One country where very little is known about the jaguar’s status and distribution is Paraguay.In their review of the jaguar’s status across its range, Swank 7 Texas Tech University, Anthony J. Giordano, May 2015 and Teer (1989) failed to acknowledge the presence of jaguars in Paraguay. While jaguars formerly were widely distributed across the country very recently, their populations in eastern Paraguayhave declined substantially. Agricultural development now dominates eastern Paraguay at the expense of formerly expansive and diverseUpper Parana Atlantic Forests (UPAF)(Cartes 2003; Huang et al. 2007). Although recent transboundary jaguar research and conservation in this region have made efforts to include Paraguay (Paviolo et al. 2008; Haag et al. 2010; De Angelo et al. 2011a, 2011b), data on jaguar populations in Paraguay’s UPAF is extremely limited, and few studies exist (e.g., Zuercher et al. 2001; Fundación Moisés Bertoni, unpub. data). Other ecological regions suffer similarly from a complete lack of information. For example, nearly one-third of the more than one million km2, heterogenous Gran Chaco ecoregion, which encompasses South America’s largest contiguousforested expanse after the Amazon Basin (Redford et al. 1990; Bucher & Huszar 1999), occupies almost two-thirds of Paraguay. Though the subject of prior surveys and research in the Bolivian and Argentine Chaco (Maffei et al. 2004; Altrichter et al. 2006; Quiroga et al. 2014), information on jaguars in the Paraguayan Chaco have largely been limited to a cursory examination of the jaguar’s diet (e.g., Taber et al. 1997). Our goalhere is to present the first formal review of the jaguar’s distribution and status in Paraguay. Weexpect to compile and review evidence of recent records of the jaguar inParaguay. Based on these records, ongoing research, and common practices in Paraguay, we discuss present threats to jaguars across the country, emphasizing the Chaco, and their implications in the context ofthe jaguar’sregional conservation needs. Finally, we recommend possible jaguar threat mitigation practices and strategies for Paraguay. METHODS We compiled all known and previously unreported records of jaguars as maintained by a variety of institutional and individual sources in Paraguay. This includes firsthand field surveys as conducted by the first author; however, the majority of reports were based on the accounts of biologists, experienced land managers, nonprofit environmental staff, and government officials. We only included reports of 8 Texas Tech University, Anthony J. Giordano, May 2015 jaguars as recently as the mid-late 1980’s, as this was when the nationalmuseum of natural history formally conducted its most recentcountry-wide biodiversity survey(Gamarra de Fox and Martin 1996). We solicited individual records from collaborators both past and present, and/or colleagues with whom our team had established personal and professional relationships, including biologists and other conservation professionals, staff at cattle-production operations, and personnel from the Secretariat of the Environment (SEAM), the federal agency charged with all environmental affairs. We also conducted a comprehensive review of published and unpublished literature, museum records, and regional checklists, and included all information obtained during the course of general biodiversity surveys conducted as part of the preparation of private land management plans. It is important to note we did not include information obtained during conversations with numerous people that make either vague or specific reference to jaguar occurrences in both broad and specific areas if they were not part of our pre-defined network. We did this to avoiddiluting data quality and to maintain consistency in the information associated with records. We mapped all recordsobtained according to the type of evidence supporting them. Evidentiary support for records was categorized broadly as (1) observations, (2) secondhand reports (“interviews”), (3) sign, and (4) a combination of both secondhand reports (interviews) and sign. “Observations” included only firsthand observations of jaguars made by us and designated conservation partners. This includes records logged during field activity by team members or other biologists in unpublished reports and notes, museum records satisfying this criterion, and camera-trap photos from various locations. “Secondhand” reports or “interviews” include conversations with other biologists, landowners, NGO staff, and agribusiness employees, as well as those conducted by staff of partner conservation institutions with whom we have had an established working relationship. “Sign” includes a variety of field markings indicative of jaguar presence, including tracks, scat, scrapings, prey remains, depredations, etc., or some combination of these. As with other evidence categories, this includes those personally observed by either one or more of our team, or via a 9 Texas Tech University, Anthony J. Giordano, May 2015 network of other qualified scientific staff based at our institutions, or those of our partners. The final “combination” category includes both some type of sign as defined above, as well as information obtained from reports in the field, typically made by land managers and knowledgeable ranch laborers. In addition, we made additional, subjective characterizations regarding the occurrence of jaguars in areasbased on ourexperience. These can broadly be categorized as (1) the probable occurrence of jaguars in areas with which we are familiar and experienced but from which records meeting our criteria were lacking (mapped as “P”), and (2) broad areas from which we lacked records and in which we are unsure (mapped as “?”) if jaguars are present (as indicated by a “?” on the map). Finally, we reviewthe threats to jaguars across the ParaguayanGran Chaco, as well as other ecoregions in the country, in the context of the distributional records we present here. We compare threats jaguars face across the different parts of Paraguay, relying on a variety of sources to assess the severity of threats, including the scientific literature, unpublished reports, regional socio-economic and political issues, existing and emerging projects, and the results of workshops and interviews. We then discuss the possible implications of these existing and emerging threats to the jaguar’s longterm persistence across Paraguay and the Chaco, and make general and specific recommendations for actions and strategies that might mitigate existing and pre-empt future threats. JAGUAR RECORDS While direct and indirect evidence for each jaguar record varied, no records were part of a field survey of jaguars with the exception of those field activities conducted by the first author. We logged a total of 132 recent independent occurrence records of jaguars across Paraguay (Table 1). The overall spatial distribution of our records suggests that jaguars are still widely-distributed across many parts of the country (Figure 1); however, the number of records and apparent status appeared to vary according to ecoregion. Observations made in the vicinity of other evidence were treated as observations only, as we felt the inclusion of less direct evidence was 10 Texas Tech University, Anthony J. Giordano, May 2015 not necessary in these circumstances. Also, in the absence of rigorous surveys, we cannot make inferences with respect to jaguar abundance or occupancy for these areas. In the Upper Parana Atlantic Forest (UPAF) ecoregion (Upper Parana Basin), we mapped 32 jaguar location records across six political departments. Two-thirds included actual observations of jaguars, while another five were based on secondhand interview records; three of the latter also included firsthand evidence of jaguar tracks. Most (n=25) records occurred at or in close proximity to protected areas, largely private reserves. It is worth noting that while we present only five records from Mbaracayu Forest Nature Reserve (MFNR), this forest is widely-accepted to host a reproducing jaguar population (Zuercher 2001, Zuercher et al. 2001; De Angelo et al. 2011b) of between 20-50 individuals (F. Ramirez, 2011 communication regarding ongoing research and unpublished data), and thus where observations are made regularly by staff and park rangers (Fundación Moisés Bertoni, multiple in-person communications made from 2005 - 2011). Unsurprisingly, the cerrado, represented by a small geographical region in northeastern Paraguay (Figure 1) and perhaps the least surveyed with respect to medium-large mammals, held the fewest number of jaguar occurrence records (n=6). Sporadic friction over land rights among the government, landowners, and settlers, particularly around protected areas (Clark 2012), is partly responsible for the area’s isolation. Five records however were observations, one of which occurred in Serranía San Luis National Park; in addition, genetic confirmation of scat samples from Paso Bravo National Park confirm their presence there (A. J. Giordano, unpub. data). Of the 27 occurrence records we report from the humid Chaco, 19 of these were actual observations. We define the humid Chaco as most of the department President Hayes (most records), but also extensive areas east of and along the Rio Paraguay in Concepcion and San Pedro (Figure 2) where a few records were unexpected. As clear boundaries are difficult to delineate with respect to the Chaco’s many ecotones, this also includes some semi-arid areas of the ‘transitional Chaco.’ With the exception of Tifunqué National Park, all of these observations occurred on non-reserve private land used for agricultural and/or cattle production, several in 11 Texas Tech University, Anthony J. Giordano, May 2015 proximity to or among private ‘conservation reserve’ easements. One interview from the department of Ñeembucú (Figures 1-2) suggested the presence of jaguars in this mixed grassland region to the south; this was unexpected, and is the most southerly recent record we have for the jaguar in Paraguay. An observation from Tifunqué National Park (Figure 3), and both tracks and an observation in areas southeast of the park, may suggest their continued presence in this area close to the Argentine border. Camera-trap photos of a male and probable female jaguar at the private reserve Toro Mocho (Figure 3) indicate jaguars are still present along the Argentine border in the transitional Chaco (Giordano et al. 2014). Informal conversations with inhabitants of the region, as well as track surveys, also confirm that jaguars are still widelydistributed, though not necessarily abundant, in the central humid Paraguayan Chaco. We collected 66 occurrence records of jaguars from the dry Chaco-Pantanal ecoregions of Paraguay (Figures 1, 3). We combined occurrence records for the dry Chaco-Pantanal, as transitional zones between the two in north-northeastern Paraguay are extensive, and their boundary generally difficult to delineate. Almost two-thirds of all records were actual observations (n=41). Of the 9 where just tracks were observed, 5 of these sign records were observed firsthand by the first author (n=1 for Teniente Enciso National Park; n=4 for Chaco Médanos National Park; Figure 3). Of the observations made at Chaco Defensores National Park (Figures 1-3), 9 were made by the first author during region-wide jaguar surveys (A. J. Giordano, unpub. data). Multiple observations of jaguars by us and our partners in the Paraguayan Pantanal at a private reserve (Los Tres Gigantes; communication by Guyra Paraguay in 2011), Fortin Patria, and other parts of this region (Figure 1, 3). Secondhand interviews comprised 6 independent records from the region that were also independent of areas where observations occurred. In addition, we encountered sign of depredations on cattle by both jaguars and pumas with some frequency across the region, though the majority of livestock carcasses had decomposed beyond our ability to determine cause-specific mortality. Finally, as evidence of jaguars constituting these records was collected opportunistically, we should caveat that there likely exists a bias of records toward 12 Texas Tech University, Anthony J. Giordano, May 2015 areas most frequently trafficked by people, where the local human population density is greater, and where our collective interests were known and thus reports more forthcoming. Conversely, large areas devoid of records may reflect either a lack of jaguar presence, or be more reflective of a lack of paved or passable roads, a low density of municipal or residential infrastructure, a lack of activity by biologists, and/or a lack of interest among residential stakeholders in reporting unsolicited jaguar records. To this last point, it is worth stating that while most observations of jaguars are memorable for each beholder, such encounters among rural agricultural stakeholders may not be an infrequent part of life; there may thus be little urgency in reporting these occurrences, particularly if these stakeholders engage in illegal retaliations. Ultimately, opportunistic records such as these can still be of practical use to planning the location or scope of surveys, and more detailed faunal investigations (Giordano et al. 2011). CURRENT THREATS TO JAGUARS Eastern Paraguay The greatest historical expanse of the Atlantic Forest biome formerly occurred across coastal and inland southeastern Brazil (Galindo-Leal & Camera 2003), which has lost as much as 99% of its original expanse (Saatchi et al 2001). Historically, a smaller portion of the overall biome, the Upper Parana Atlantic Forest (UPAF) ranged inland south and west to northeastern Argentina and eastern Paraguay. By 1981, approximately 6% of the farmers in eastern Paraguay owned nearly 80% of the land, which was largely dominated by this forest (Weisskoff 1992). By 2000, approximately 80% of the intensive deforestation the region experienced was a result of these few agribusinesses converting large holdings to soybean production and to a lesser extent, sugar and cotton (Nickson 1981; Rios & Zardini 1989; Macedo & Cartes 2003; Huang et al. 2007, 2009; Yanosky 2012). Similarly, a small portion of the cerrado biome, nearly all of which occurs in Brazil today (Klink & Machado 2005), historically extended into southeastern Bolivia,and northeast Paraguay east of the Paraguay River in the Departments of Concepcion and Amambay, and eastern Canindeyú (Figures 2-3). As recently as the mid-1980’s, nearly half of eastern 13 Texas Tech University, Anthony J. Giordano, May 2015 Paraguay was still dominated by this habitat and its transitional zones, an area 20% greater than that occupied by the UPAF(Zardini 1993). Today, there is even less cerrado in eastern Paraguay than UPAF. Unsurprisingly, jaguars have largely disappeared from the majority of Paraguay east of the Rio Paraguay. Current UPAF strongholds for the jaguar include Mbaracayu Forest Nature Reserve (~644 km2), two Itaipu Nature Reserves (97 – 124 km2), and Morombi Nature Reserve (~250 km2), which are among the larger or most secure Atlantic Forest remnants in Paraguay (Zuercher 2001, Clark 2012) (Figure 3). However, our records are noteworthy because they suggest jaguars may still persist in areas outside of current stronghold protected areas in this region. While most forest fragments and protected areas in this regionmay be too small to maintain jaguar populations in the long-term, it is unclear how many are being used by jaguars and in what capacity (e.g., sub-adult males searching for territory). A more critical situation may exist for jaguars in the Paraguayan cerrado. Aside from the portion of cerrado occupying the Mbaracayu region, our records indicate the jaguar occurs in Paso Bravo National Park (~1030 km2) and Serranía San Luis National Park (~103 km2)in northern Concepcion (Figures 2-3), the former of which may contain the largest, contiguous portion of cerrado in the country. As with the UPAF, we have records of jaguars in this region outside of protected areas. However as also with the UPAF, it is very possible that several or all of these records represent non-resident individuals, and targeted surveys in this region as in many parts of the UPAF are warranted. Today, persistent threats to the viability, expansion, and restoration of jaguar populations remain in eastern Paraguay. In the UPAF biome, illegal activities continue to degrade, fragment, and encroach upon the borders of protected areas and their buffer zones (Huang et al. 2007, 2009; Clark 2012). These activities, which have their origins in historical land rights disputes, range from illegal timber harvest, agricultural encroachment, the harvest of medicinal plants for subsistence use, cattlegrazing, human-caused fires, re-settlement of rural communities inside the park, and poaching (Clark 2012, Yanosky 2012). San Rafael National Park (~780 km2), which encompasses an important regional watershed region of the Rio Parana and is the 14 Texas Tech University, Anthony J. Giordano, May 2015 largest Paraguayan protected area containing UPAF, typifies these problems in many ways. Similar threats, as well as soil degradation and mineral extraction, plague many other protected areas in eastern Paraguay, including Yvytyrusu Resource Management Reserve (~240 km2), Caazapá National Park (~123 km2), and Ypeti Nature Reserve (~136 km2) (Figure 3). Yvytyrusu currently hosts 1000 or so families within its boundaries, including small-holding farmers, a major reason it has not been declared a national park. Neither it, nor Caazapá or San Rafael National Parks, are believed to host jaguars (direct communications by SEAM, F. Ramirez, and J. F. Cartes in 2011, regarding previous surveys and ongoing research). Jaguars may however still use Ypeti Nature Reserve, which contains a mosaic of UPAF, wetlands, and grassland (Clark 2012). In contrast, pumas appear to use many of these areas (Clark 2012; direct communication in 2011 by F. Ramirez, Guyra Paraguay, and Fundación Moises Bertoni regarding unpublished surveys and interviews). In the cerrado, frequent burning of grassland to create new pasture with invasive grasses, as well as constant human re-settlement and encroachment with cattle, must be considered a major threat to the entire biome in Paraguay. In addition, poaching of mammals, deforestation, illegal extraction of timber for charcoal and other uses, harvesting of medicinal plants, small-scale encroachment of agricultural crops, and a number of proposed water projects represent threats to board parts of the region (Robbins 1999; Clark 2012), including both National Parks. Increased isolation of protected areas and viable cerrado habitat is also a concern in this region, and for much of the country, a lack of adequate staffing and infrastructure for the country’s ‘public’ protected areas, as well as its private‘reserve’ holdings, are a major challenge to overcome (Yanosky &Cabrera 2003; Huang et al. 2009; Clark 2012; Yanosky 2012). Comparably less is known about the status of jaguars in southeastern Paraguay, and this region may be the least known with respect to the jaguar’s distribution and ecology. Similar threats exist to ecosystems in this area as exist elsewhere, including overgrazing, burning for new pasture, encroachment of non-native grass species, and the planting of non-native trees (Clark 2012). Our records suggest that jaguars may 15 Texas Tech University, Anthony J. Giordano, May 2015 still be using Atlantic and gallery forest fragments, and humid palm savanna (i.e., “Chaco”) in this region. However, as with other parts of eastern Paraguay, it is unlikely that much reproduction is occurring, and more probable that these individuals represent temporary residents, or animals looking for a new territory. Gran Chaco The Gran Chaco once covered 1.2 million km2 of northern Argentina (~46%), northern and westernmost Paraguay (~32%), south-eastern Bolivia (~15%), and a small portion in south-central Brazil (<7%) along Mato Grosso do Sul (Prado 1993; Zardini 1993; Pennington et al. 2000). Historically jaguars ranged across the entire Gran Chaco ecoregion, including central Argentina (Caso et al. 2008; Rumiz et al. 2011; Di Bitetti et al, in press). Until recently, the Gran Chaco was considered one of the last great expanses of remote and relatively ‘undisturbed’ wilderness regions in South America (Verschuren 1980; Hannah et al. 1994, 1995; Redford et al. 1990; Yanosky 2012). Today, estimates of annual deforestation rates for the region (e.g., 1.5 - 2.5%, ~2.2%) now exceed the average loss for all global forests since the middle of the 19th century (Rowe et al. 1992; Steininger et al. 2001; Zak et al. 2004; Foley et al. 2005). In Paraguay, this has largely been driven by the high cost of beef, which has resulted in both expansive and intensive clearing of forest for cattle-ranching (Caldas et al. 2013; Yanosky 2012, 2013). Whereas surveys in the southern Bolivian Chaco close to Paraguay indicate that jaguar populations may be relatively secure there (Maffei et al. 2004), more recent surveys in the Argentine Chaco suggest that the species is critically endangered and nearly extinct (Quiroga et al. 2014). In contrast, the population status of jaguars in the Paraguayan Chaco has been less well known, and the socioeconomic context for drivers of deforestation in the Paraguayan Chacois somewhat different there than in the Argentine or Bolivian Chaco. Based on the records we include here, jaguars do appear to range widely across both the humid and dry Chaco, and the Paraguayan Pantanal. Across the humid (low) Chaco, jaguar occurrences appear to be more fragmented; this is unsurprising, as this region has a longer history of ranching and development than the dry (high) Chaco (Caldas et al. 2013). Of increasing concern 16 Texas Tech University, Anthony J. Giordano, May 2015 however is that habitat conversion and fragmentation are now impacting both the dry Chaco and Pantanal, particularly in transboundary regions (i.e., Paraguay’s northeastern most borders with Brazil and Bolivia, and central western border with Argentina). This includes semi-arid transitional and dry Chaco forest contiguous with the Argentinean frontier, as well as forests close to Bolivia’s Chaco-Pantanal transition zone, the latter of which may be undergoing some of the highest deforestation rates in the Paraguayan Chaco and Pantanal (several direct communications with Guyra Paraguay from 2011-2013 regarding their ongoing monitoring of deforestationusing remote imagery). The accelerating pattern of land use changes across the approximately 200,000 km2 Brazilian Pantanal, of which jaguars occupy nearly two-thirds, present urgent threats to the species there as well (Cavalcanti et al. 2012). The severity of these threats notwithstanding, both the Gran Chaco and the Pantanal are considered priority regions for long-term jaguar population persistence (Sanderson et al. 2002; Rabinowitz & Zeller 2010); unlike in Paraguay however, jaguars have already been the subject of considerable research considerable attention in the Brazilian Pantanal (e.g., Schaller & Crawshaw 1980; Crawshaw & Quigley 1991; Quigley & Crawshaw 1992; Azevedo & Murray 2007; Cavalcanti & Gese 2009). Together, the Gran Chaco of Paraguay and Bolivia, and the Brazilian Pantanal, encompass two of the largest Jaguar Conservation Units (JCU’s) (c.f. Sanderson et al. 2002; Rabinowitz &Zeller 2010) outside of the Amazon Basin. Both northern Paraguay and southern Bolivia are thus critical links to broad-scale connectivity for jaguars across this entire region, including the entire expanse of the Gran Chaco, and between the Chaco and Pantanal biomes. As has been reported elsewhere across Latin America, deforestation and land use practices leading to conflict with jaguars over livestock, in conjunction with a steady decline in jaguar habitat and prey abundance, likely represent the most severe and immediate threat to the jaguar across the Paraguayan Chaco. Legal deforestation thresholds are routinely exceeded, new licenses are still being issued for land-clearing, and mandatory land management plans are not always properly prepared or filed (Carron 2000,Yanosky 2012). Not surprisingly, we recorded the fewest records (n=4) 17 Texas Tech University, Anthony J. Giordano, May 2015 from the vast majority of Paraguay’s largest department, Boquerón (Figure 2), which has experienced some of the highest deforestation rates in between the 1990’s and 2000’s (MAG 2011). The remaining records (n=13) were associated with the large protected areas in the department’s far north (eg., Chaco Medanos National Park, Enciso National Park; Figure 3). Lack of enforcement of deforestation laws, “foreignization” of land and absentee landowners, lack of guidelines regarding the spatial arrangement or configuration of cutting practices, and loopholes in deforestation laws that permit additional clearcutting after land is resold, all represent land management challenges to protecting Gran Chaco habitat for the jaguar in Paraguay. While many of ultimate drivers of jaguar population declines are complex and interactive, the most serious and widespread direct threat to jaguars relates to retribution for, and perceived preemption of, depredation of livestock (Hoogesteijn & Hoogesteijn 2010a). The intensity of human-jaguar conflict in an area is either precipitated or exacerbated by the loss and fragmentation of habitat, and the concurrent elimination of potential jaguar prey (Rabinowitz 1995, 2005). Declines in prey populations are caused not only by habitat loss but also hunting by local people, which quite often prefer the same medium-large mammal prey as jaguars,and with deforestation,are suddenly afforded increased access to previously-remote areas (Ojasti 1984; Jorgenson & Redford 1993; Cullen et al. 2000; Leite & Galvao2002; Crawshaw 2003;Altrichter 2005, 2006). Also, the prevalent misconception among landowners and ranch employees that every jaguar is a probable depredator of cattle drives many to take ‘preventative measures’ in protecting their livestock. This has resulted in a culture of opportunistically shooting jaguars on sight (Rabinowitz 1986; Hoogesteijn et al. 1993; Rabinowitz 1995), particularly in those regions where landowners have previously experienced some level of conflict. The shooting of jaguars in both of these contexts has the potential to yield more conflicts rather than remedy or prevent problems, as many predators are wounded in the jaw, head, or body instead of killed and then become less able to take their natural prey (Rabinowitz 2005; Hoogesteijn & Hoogesteijn 2010a). 18 Texas Tech University, Anthony J. Giordano, May 2015 Two general scenarios in particular can lead to greater conflict with jaguars (Hoogesteijn & Hoogesteijn 2005), variations of both of which can be encountered in the Paraguayan Chaco. Expansive artificial or introduced pastures might be broadly categorized as one such scenario. Such pastures are created through extensive deforestation on large land holdings, frequently large-scale cattle agribusinesses, and may represent what is typically thought of as the traditional deforestation problem with respect to jaguars. These operations often function below critical thresholds of forest area and jaguar prey abundance, which cause jaguars to avoid the vicinity of open clearings, including introduced pastures, and restrict themselves to the remaining forest and prey (Rabinowitz & Nottingham 1986;Rabinowitz 1995; Hoogesteijn& Hoogesteijn 2000b). Thus as more forest is lost, the less habitat and prey there is for jaguars. A second problem scenario however might be broadly categorized as native or natural range, where cattle range freely across mostly native vegetation, and little ‘active’ pasture is maintained. Under this scenario, deforestation or active clearing on a ranch is either substantially less than the prior scenario, or completely non-existent; this is generally irrespective of deforestation patterns as perpetrated by neighbors and others in the region, which can be extensive or vary in intensity across a larger landscape mosaic. Ultimately, it is deforestation and the creation of pasture with little planning or coordination with neighbors in maintaining native forest, as well as a lack of active livestock management, that probably leads to greater conflict-related problems with jaguars in the Paraguayan Chaco and therefore the most important proximate threat. MANAGEMENT RECOMMENDATIONS Unlike eastern Paraguay, the Gran Chaco still has potential to represent a stronghold for jaguar populations, in spite of accelerating deforestation. However, this would mean reconciling the current socioeconomic drivers of expanding livestock production and its deleterious impacts on the region’s forests to immediately address the larger overarching threats of deforestation and direct persecution. Record high beef prices, which continue to trend upwards, are changing the overall cost structure related to the expenses of clearing of vegetation. More policies to incentivize the 19 Texas Tech University, Anthony J. Giordano, May 2015 conservation of greater expanses of habitat, as well as a greater capacity for enforcing existing legislation, are essential moving forward. A greater support system for landowners experiencing conflict with jaguars and pumas, or for those willing to be proactive in pre-empting conflict, might demonstrate the type of commitment needed to begin changing attitudes among cattle production operations experiencing economic losses. Information regarding the importance and role of jaguars and other predators to natural ecosystems, as well as the services natural ecosystems provide (i.e., mitigating drought), should be integrated into national and local education programs, and intensive public awareness campaigns. Landowners should be encouraged to explore alternative land uses compatible with cattle production, yet reliant on the conservation of wildlife and natural habitat; this includes organized ecotourism and the hosting of international research teams, both of which are a promising means to enhance the revenue their land might generate. New legislation offering jaguars added protection through the SEAM, in particular on private land, as well as the potential “incentivization” of reporting jaguars killed illegally, should be accompanied by routine enforcement, even in the most remote regions. The declaration of more inviolate public protected areas in Paraguay, accompanied by adequate supporting staff and the infrastructure essential to staff duties for those that exist now, should be part of any comprehensive approach to maintain national and ecoregional connectivity among jaguar populations. Collectively, creative policies in the rural Chaco can lead to incentivized, sustainable development and continued cattle production, greater diversification of land use and economic growth, the exploration of international carbon and ecosystem services markets, maintenance of ecosystem functioning, a reduction in conflict with jaguars, and a change in public attitudes toward the continent’s largest predator. 20 Texas Tech University, Anthony J. Giordano, May 2015 REFERENCES Altrichter M. 2005. The sustainability of subsistence hunting of peccaries in the Argentine Chaco. Biol Conserv. 126(3):351-362. Altrichter M. 2006. Wildlife in the life of local people of the semi-arid Argentina Chaco. Biodivers Conserv. 15(8):2719-2736. Astete S, Sollman R, Silveira L. 2008. Comparative ecology of jaguars in Brazil. Cat News, Special Issue 4:9-14. Azevedo FCCd, Murray DL. 2007. Spatial organization and food habits of jaguars (Panthera onca) in a floodplain forest. Biol Conserv. 137(3):391-402. Briones-Salas M, Lavariega M C, Lira-Torres I. 2012. Distribución actual y potencial del jaguar (Panthera onca) en Oaxaca, Mexico. Rev Mex Biodivers. 83(1):246-257. Bucher EH, Huszar PC. 1999. Sustainable management of the Gran Chaco of South America: ecological promise and economic constraints. J Environ Manage. 57(2):99-108. Caldas MM, Goodin D, Sherwood S, Campos Krauer JM, and Wisely SM. 2013. Land-cover change in the Paraguayan Chaco: 2000-2011. J Land Use Sci, DOI: 10.1080/1747423X.2013.807314. Carron JM. 2000. The Pantanal of Paraguay. In: Swarts FA, editor. The Pantanal. St. Paul (MN): Paragon House Publishers. p. 55-67. Cartes JL. 2003. Brief history of conservation in the interior Atlantic Forest. In: Galindo-Leal C, Camera IdG, editors. The Atlantic Forest of South America: biodiversity status, threats, and outlook. Washington DC: Island Press. p. 269287. Caso A, Lopez-Gonazlez CA, Payan E, Eizirik E, Oliveira Td, Leite-Pitman R, Kelly M, Valderrama C. 2008. Panthera onca. IUCN Red List of Threatened Species Version 2013.2 [Internet]. [cited 2013 Oct 15].. Available from: http://www.iucnredlist.org/. Cavalcanti SMC, Gese, EM. 2009. Spatial ecology and social interactions of jaguars (Panthera onca) in the southern Pantanal, Brazil. J Mammal. 90(4):935-945. 21 Texas Tech University, Anthony J. Giordano, May 2015 Charre-Medellin JF, Monterrubio-Rico TC, Botello FJ, Leon-Paniagua L, Nunez R. 2013. First records of jaguar (Panthera onca) from the state of Michoacan, Mexico. Southwestern Nat. 58(2):264-268. Clark PT. 2012. Guide to Paraguay’s National Parks and Other Protected Areas. Revised 2nd ed. Asuncion (Paraguay). Published by Peter T. Clark. Crawshaw PG. 2003. A personal view on the depredation of domestic animals by large cats in Brazil. Nat Conservacao. 1(1):71-73. Crawshaw PG, Quigley HB. 1991. Jaguar spacing, activity, and habitat use in a seasonally flooded environment in Brazil. J Zool. 223(3):357-370. Cullen Jr. L., Bodmer RE, Padua, CV. 2000. Effects of hunting in habitat fragments of the Atlantic forests, Brazil. Biol Conserv. 95(1):49-56. de la Torre J., Medellin RA. 2011. Jaguars Panthera onca in the greater Lacandona Ecosystem, Chiapas, Mexico: population estimates and future prospects. Oryx 45(4):546-553. De Angelo C, Paviolo A, Di Bitetti M. 2011a. Differential impact of landscape transformation on pumas (Puma concolor) and jaguars (Panthera onca) in the Upper Parana Atlantic Forest. Divers Distrib. 17(3):422-436. De Angelo C, Paviolo A, Rode D, Cullen Jr L, Sana D, Abreu KC, da Silva MX, Bertrand A-S, Haag T, Lima F, Rinaldi AR, Fernandez S, Ramirez F, Velazquez M, Corio C, Hasson F, Di Bitetti MS. 2011b. Participatory networks for large-scale monitoring of large carnivores: pumas and jaguars of the Upper Paraná Atlantic Forest. Oryx 45(4):534-545. Di Bitteti M, Quiroga V, De Angelo C, Altrichter M, Paviolo A, Cuyckens E, Boaglio G, Earnhardt JM, Lonsdorf E. Forthcoming 2015. El yaguareté (Panthera onca) en la Argentina: situación poblacional, amenazas, y acciones para su conservación. In: Ramadori D, Porini G, editors. Manejo de Fauna en la Argentina. Acciones para la Conservacion de Especies Amenazadas. Buenos Aires (Argentina): Direccion de Fauna de la Nacion Argentina, Secretaria de Ambiente la Nacion Editora. 22 Texas Tech University, Anthony J. Giordano, May 2015 Euwe RA, Weterings M, Dummer I, Polisar J, Salom-Perez R. 2013. Optimizing jaguar prey occupancy in Neotropical oil palm plantations: a case study in Nicaragua. Poster presented at: Annual Meeting of the Association for Tropical Biology and Conservation & Organization for Tropical Studies; San Jose, Costa Rica. Foley JA, Defries R, Asner GP, Barford C, Bonan G, Carpenter SR, Chapin III FS, Coe MT, Daily GC, Gibbs HK, Helkowski JS, Holloway T, Howard EA, Kucharik CJ, Monfreda C, Patz J, Prentice IC, Ramankutty N, Snyder PK. 2005. Global consequences of land use. Science 309(5734):570-574. Furtado MM, Carillo-Percastegui SE, Jacomo ATA, Powell G, Silveira L, Vynne C, Sollmann R. 2008. Studying jaguars in the wild: past experiences and future perspectives. Cat News, Special Issue 4:41-47. Galindo-Leal C., Camera IdG, editors. 2003. The Atlantic Forest of South America: biodiversity status, threats, and outlook. Washington DC: Island Press. Gamarra IdF, Martin AJ. 1996. Mastozoologia. In: OR Martinez, editor. Colecciones de Flora y Fauna del Museo Nacional de Historia Natural del Paraguay. Asuncion (Paraguay): Museo Nacional de Historia Natural del Paraguay, SEAM. p. 469-573. Giordano A J, Carrera R, Ballard W. 2011. Assessing the credibility of jaguarundi observations using diagnostic criteria and witness qualification. Hum Dimens Wildl. 16(5):360-367. Giordano AJ, Mujica MN, Ramirez F, Nielsen C. 2014. Jaguar (Panthera onca) records from the southcentral Paraguayan Chaco. Cat News 60:38-40. Grigione MM, Menke K, Lopez-Gonzalez C, List R, Banda A, Carrera J, Carrera R, Giordano AJ, Morrison J, Sternberg M, Thomas R, Van Pelt B. 2009. Identifying potential conservation areas for felids in the USA and Mexico: integrating reliable knowledge across an international border. Oryx 43(1):7886. Haag T, Santos AS, Sana DA, Morato RG, Cullen Jr L, Crawshaw Jr PG, De Angelo C, Di Bitetti MS, Salzano FM, Eizirik E. 2010. The effect of habitat 23 Texas Tech University, Anthony J. Giordano, May 2015 fragmentation on the genetic structure of a top predator: loss of diversity and high differentiation among remnant populations of Atlantic Forest jaguars. Mol Ecol. 19(22):4906-4921. Hannah L, Lohse D, Hutchinson C, Carr JL, Lankerani A. 1994. A preliminary inventory of human disturbance of world ecosystems. Ambio 23(4/5):246-250. Hannah L, Carr JL, Lankerani A. 1995. Human disturbance and natural habitat: a biome level analysis of a global data set. Biodivers Conserv 4(2):128-155. Hoogesteijn R, Mondolfi E. 1993. Jaguar predation and conservation: cattle mortality by felines on three ranches in the Venezuelan Llanos. Mammals as Predators. Proc Symp Zool Soc London. 65:391-407. Hoogesteijn R., Hoogesteijn A. 2005. Manual sobre os problemas de predacao causados por oncas em gado de corte. Campo Grande (Brazil): Wildlife Conservation Society. Hoogesteijn A, Hoogesteijn R. 2010a. Cattle ranching and biodiversity conservation as allies in South America’s flooded savannas. Great Plains Res. 20(1):37-50. Hoogesteijn R, Hoogesteijn A. 2010b. Conserving wild felids in humanized landscapes: strategies for reducing conflicts between jaguars and cattle. Wild Felid Mon. 3(2):1,9-13. Huang C, Kim S, Alstatt A, Townshend JRG, Davis P, Song K, Tucker CJ, Rodas O, Yanosky A, Clay R, Musinsky J. 2007. Rapid loss of Paraguay’s Atlantic forest and the status of protected areas – A Landsat assessment. Remote Sens Environ. 106(4):460-466. Huang C, Kim S, Song K, Townshend JRG, Davis P, Alstatt A, Rodas O, Yanosky A, Clay R, Tucker CJ, Musinsky J. 2009. Assessment of Paraguay’s forest cover change using Landsat observation. Global Planet Change. 67(1):1-12. Jorgenson JP, Redford KH. 1993. Humans and big cats as predators in the Neotropics. Mammals as predators. Proc Symp Zool Soc London. 65:367-390. Karanth KU. 1995. Estimating tiger Panthera tigris populations populations from camera-trap data using capture-recapture models. Biol Conserv. 71(3):333338. 24 Texas Tech University, Anthony J. Giordano, May 2015 Karanth KU, Nichols JD. 1998. Estimation of tiger densities in India using photographic captures and recaptures. Ecology 79(8):2852-2862. Klink CA, Machado RB. 2005. Conservation of the Brazilian Cerrado. Conserv Biol. 19(3):707-713. Leite MRP, Galvao F. 2002. El jaguar, el puma, y el hombre en tres areas protegidas del bosque atlantico costero do Parana, Brazil. In: Medellin RA, Equihua C, Chetkiewicz CL, Crawshaw PG, Rabinowitz AR, Redford KH, Robinson JG, Sanderson EW, Taber AB, editors. El Jaguar en el Nuevo Milenio. Mexico City (MX): Fondo de Cultural Economica, Universidad Nacional Autonoma de Mexico/ Wildlife Conservation Society. p. 237-250. Macedo AM, Cartes JL. 2003. Socieconomic drivers in the Interior Atlantic Forest. In: Galindo-Leal C, Camara IdG, editors. The Atlantic Forest of South America: Biodiversity status, threats, and outlook. Washington DC: Island Press. p. 310324. Maffei L, Cuellar E, Noss A. 2004. One thousand jaguars in Bolivia’s Chaco? Camera trapping in the Kaa-Iya National Park. J Zool. 262(3):295-304. McCain EB, Childs JL. 2008. Evidence of resident jaguars (Panthera onca) in the southwestern United States and the implications for conservation. J Mammal. 89(1):1-10. [MAG] Ministerio de Agricultura y Ganaderia (Paraguay). 2011. Produccion Agropecuaria: Sintesis Estadisticas (Zafra Agricola 2010/ 2011). Asuncion (Paraguay), Omega Editoria SRL. Monroy-Vilchis O, Sanchez O, Aguillera-Reyes U, Suarez P, Urios V. 2008. Jaguar (Panthera onca) in the state of Mexico. Southwestern Nat. 53(4):535-539. Navarro-Serment CJ, Lopez-Gonzalez CA, Gallo-Reynoso J.-P. 2005. Occurrence of jaguar (Panthera onca) in Sinaloa, Mexico. Southwestern Nat. 50(1):102-106. Nickson RA. 1981. Brazilian colonization of the eastern border region of Paraguay. J Lat Am Stud. 13(1):111-131. Ojasti L.1984. Hunting and conservation of mammals in Latin America. Acta Zool Fenn. 172:177-181. 25 Texas Tech University, Anthony J. Giordano, May 2015 Paviolo A, De Angelo C, Di Blanco Y, Di Bitetti M. 2008. Jaguar population decline in the Upper Parana Atlantic Forest of Argentina and Brazil. Oryx 42(4):554561. Petracca LS, Ramirez-Bravo OE, Hernandez-Santin L. 2013. Occupancy estimation of jaguar (Panthera onca) to assess the value of east-central Mexico as a jaguar corridor. Oryx 48(1):133-140. Quigley HB, Crawshaw PG. 1992. A conservation plan for the jaguar (Panthera onca) in the Pantanal region of Brazil. Biol Conserv. 61(3):149-157. Quiroga VA, Boagio GI, Noss AJ, Di Bitetti MS. 2014. Critical population status of the jaguar (Panthera onca) in the Argentina Chaco: camera-trap surveys suggest recent collapse and imminent regional extinction. Oryx 48(1):141-148. Rabinowitz AR. 1986. Jaguar predation on domestic livestock in Belize. Wildlife Soc B. 14(2):170-174. Rabinowitz AR, Nottingham B. 1986. Ecology and behavior of the jaguar (Panthera onca) in Belize, Central America. J Zool. 210(1):149-159. Rabinowitz A. 1995. Jaguar conflict and conservation: a strategy for the future. In: Bissonette JA, Krausman PR, editors. Integrating People and Wildlife for a Sustainable Future. Bethesda (MD): The Wildlife Society. p. 394-397. Rabinowitz A. 2005. Jaguars and livestock: living with the world’s largest cat. People and Wildlife: Conflict or Coexistence? In: Woodroffe R, Thirgood S, Rabinowitz A, editors. Cambridge (UK): Cambridge University Press. p. 278285. Rabinowitz A, Zeller K. 2010. A range-wide model of landscape connectivity for the jaguar, Panthera onca. Biol Conserv. 143(4):939-945. Redford KH, Taber AB, Simonetti JA. 1990. There is more to biodiversity than the tropical rain forests. Conserv Biol. 4(3):328-330. Rios E, Zardini E. 1989. Conservation of Biological Diversity in Paraguay. Conserv Biol. 3(2)118-120. 26 Texas Tech University, Anthony J. Giordano, May 2015 Robbins MB, Faucet RC, Rice NH. 1999. Avifauna of a Paraguayan Cerrado locality: Parque Nacional Serrania San Luis, Depto. Concepcion. Wilson Bull. 111(2):216-228. Rosas-Rosas OC, Lopez-Soto JH. 2002. Distribucion y estado de conservacion del jaguar en Nuevo Leon, Mexico. In: Medellin RA, Equihua C, Chetkiewicz CL, Crawshaw PG, Rabinowitz AR, Redford KH, Robinson JG, Sanderson EW, Taber AB, editors. El Jaguar en el Nuevo Milenio. Mexico City (MX): Fondo de Cultural Economica, Universidad Nacional Autonoma de Mexico/ Wildlife Conservation Society. p. 393-401. Rowe R, Sharma NP, Browder J. 1992. Deforestation: problems, causes, and concerns. In: Sharma NP, editor. Managing the world’s forests: looking for balance between conservation and development. Iowa (USA): Kendall/ Hunt. p. 33-45. Rumiz DI, Polisar J, Maffei L. 2011. El futuro del jaguar en el Gran Chaco – Situacion en Bolivia, Paraguay, and Argentina. Santa Cruz (Bolivia): Wildlife Conservation Society. Saatchi S, Agosti D, Alger K, Delabie J, Musinsky J. 2001. Examining fragmentation and loss of primary forest in the southern Bahian Atlantic Forest of Brazil with radar imagery. Conserv Biol. 15(4):867-875. Sanderson EW, Redford KH, Chetkiewicz C-LB, Medellin RA, Rabinowitz AR, Robinson JG, Taber AB. 2002. Planning to save a species: the jaguar as a model. Conserv Biol. 16(1):58-72. Schaller GB, Crawshaw PG. 1980. Movement patterns of the jaguar. Biotropica 12(3):161-168. Silveira L, Boulhosa R, Astete S, de Almeida Jacomo AT. 2008. Management of domestic livestock predation by jaguars in Brazil. Cat News Special Issue 4:26-30. Silveira L, de Almeida Jacomo AT, Astete S, Sollman R, Torres NM, Furtado MM, Marinho-Filho J. 2009. Density of the Near Threatened jaguar Panthera onca in the caatinga of north-eastern Brazil. Oryx 44(1):104-109. 27 Texas Tech University, Anthony J. Giordano, May 2015 Silver SC, Ostro LET, Marsh LK, Maffei L, Noss A, Kelly MJ, Wallace RB, Gomez H, Crespo GA. 2004. The use of camera traps for estimating jaguar (Panthera onca) abundance and density using capture/recapture analysis. Oryx 38(2):148154. Soisalo M., Cavalcanti S. 2006. Estimating the density of a jaguar population in the Brazilian Pantanal using camera-traps and capture-recapture sampling in combination with GPS radio-telemetry. Biol Conserv. 129(4):487-496. Steininger MK, Tucker CJ, Ersts P, Killeen TJ, Villegas Z, Hecht SB. 2001. Clearance and fragmentation of tropical deciduous forest in the Tierras Bajas, Santa Cruz, Bolivia. Conserv Biol. 15(4):856-866. Swank RK, Teer JG. 1989. Status of the jaguar – 1987. Oryx 23(1):14-21. Taber AB, Novarro AJ, Neris N, Colman FH. 1997. The food habits of the sympatric jaguar and puma in the Paraguayan Chaco. Biotropica 29(2):204-213. Verschuren J. 1980. Saving Paraguay’s Wilderness. Oryx 15(5):465-470. Villordo-Galvan JA, Rosas-Rosas OC, Clemente-Sanchez F, Martinez-Montoya JF, Tarango-Arambula LA, Mendoza-Martinez G, Sanchez-Hermosillo MD, Bender LC. 2010. The Jaguar in San Luis Potosi, Mexico. Southwestern Nat. 55(3):394-402. Wallace RB, Gomez H, Ayala G, Espinoza F. 2003. Camera trapping for jaguar (Panthera onca) in the Tuichi Valley, Bolivia. Mastozool Neotrop. 10(1):133139. Weisskoff R. 1992. The Paraguayan agro-export model of development. World Dev. 20(10):1531-1540. Yanosky A. 2012. The challenge of conserving a natural Chaco habitat in the face of severe deforestation pressure and human development needs. In: Lambert P, Nickson A, editors. The Paraguay Reader: History, Culture, Politics (The Latin America Readers). Durham (NC): Duke University Press. p. 376-382. Yanosky A. 2013. Paraguay’s challenge of conserving natural habitats and biodiversity with global markets demanding forest products. In: Sodhi NS, 28 Texas Tech University, Anthony J. Giordano, May 2015 Gipson L, Raven PH, editors. Conservation Biology: Voices from the Tropics. Hoboken (NJ): John Wiley and Sons Ltd. p.113-119. Yanosky A, Cabrera E. 2003. Conservation capacity in the interior Atlantic forest of Paraguay. In: Galindo-Leal C, Camera IdG, editors. The Atlantic Forest of South America: biodiversity status, threats, and outlook. Washington DC: Island Press. p. 328-354. Zak MR, Cabido M, Hodgson JG. 2004. Do subtropical forests in the Gran Chaco, Argentina have a future? Biol Conserv. 120(4):589-598. Zardini EM. 1993. Paraguay’s floristic inventory. Res Explor. 9(1):128-131. Zuercher G. 2001. The ecological role of the bush dog (Speothos venaticus) as part of the mammalian predator community in the interior Atlantic forest of Paraguay [dissertation]. [Manhattan (KS)]: Kansas State University. Zuercher GL, Gipson PS, Hill K. 2001. A predator-habitat assessment for felids in the inland Atlantic Forest of eastern Paraguay: a preliminary analysis. Endanger Species Up. 18(4):115-119. 29 Texas Tech University, Anthony J. Giordano, May 2015 Figure 1.1 Map depicting jaguar records in Paraguay with respect to the country’s ecoregions and protected areas. Legend: direct observations (dark circles), interview records and field sign (open circles with X’s), field sign alone (X’s), and interviews alone (open circles). Anecdotal (secondhand) evidence and reports with no additional information are indicated by an “a”, while “?” apply to broad areas where evidence of jaguars is completely lacking. 30 Texas Tech University, Anthony J. Giordano, May 2015 31 Texas Tech University, Anthony J. Giordano, May 2015 Figure 1.2. Map of Paraguay’s political departments. The Gran Chaco occupies Presidente Hayes, Boqueron, and Alto Paraguay; the dark shape represents the location of Chaco Defensores National Park to scale at 7,800 km2 in area. 32 Texas Tech University, Anthony J. Giordano, May 2015 33 Texas Tech University, Anthony J. Giordano, May 2015 Figure 1.3.Map of protected areas with approximate extent of ecological regions. This map depicts the Chaco-Pantanal transitional zones as “Chaco Humedo” and also notes the pockets of cerrado along the extreme northern border (i.e., ‘Chovereca’ region) with Bolivia in the dry Chaco and Pantanal. Excerpted and reproduced with permission of Peter T. Clark ( © 2014). 34 Texas Tech University, Anthony J. Giordano, May 2015 35 Texas Tech University, Anthony J. Giordano, May 2015 Table 1.1 Summary of jaguar records across Paraguay’s ecoregions by evidence Observations Interviews Sign Interviews + Sign TOTAL Atlantic Forest 21 5 4 2 32 Cerrado Humid Chaco Dry ChacoPantanal TOTAL 5 19 1 3 0 4 1 1 7 27 41 86 6 15 9 17 10 14 66 132 Ecoregion 36 Texas Tech University, Anthony J. Giordano, May 2015 CHAPTER III ADAPTATION OF NONINVASIVE GENETIC TECHNIQUES FOR IDENTIFYING JAGUARS AND PUMAS IN THE GRAN CHACO: PRACTICAL CONSIDERATIONS AND IMPLICATIONS ABSTRACT Large carnivores generally possess ecological characteristics (e.g., cryptic, low density) more amenable to indirect (e.g., track/sign counts, interviews) or remote (e.g., camera-trapping) methods of survey and field investigation. Noninvasive Genetic Sampling (NGS) may present analternative to traditional camera-trapping and less robust methods, as well as hold the potential to yield more information on the sampled population. In certain tropical ecoregions however, it is unclear if NGS can be effective for technical and logistical reasons. We collected 554 complete scat (fecal) samples suspected of originating from large felids in the Paraguayan Chaco from 2008-2010. After PCR amplification ofthe ATP-6 mtDNA nucleotide sequence using primers optimized for differentiating jaguars and pumas, we identified 54% of samples as jaguar and 34% as puma. We found no relationship between either initial fecal dry volume or long-term storage andsuccessful species identification; however, human-introduced variability, time interval between DNA extraction and PCR amplification procedures, field environment, and initial storage techniques, may all influence species identification success. PCR amplification with amelogenin primers optimized for felid sex identification confirmed the sex of 77% of large felid samples. We recorded a near 1:1 sex ratio of puma samples collected systematically at “Defenders of the Chaco” National Park. In contrast, we recorded a near 5:1 ratio of male to female jaguar scat samples during this same effort. We confirm the efficacy of ATP-6 primers in identifying and differentiating jaguars and pumas in the Chaco, and conclude that additional extraction and PCR amplification efforts for samples initially failing to yield an identification may ultimately help improveoverall success. 37 Texas Tech University, Anthony J. Giordano, May 2015 INTRODUCTION The cryptic nature of many medium and large-sized terrestrial forest mammal present practical and logistical challenges to their study (Thompson 2004; O’Connell et al. 2011). In tropical forests, sampling design constraints can be further compounded by relatively low visibility, high topographical diversity, and low population densities, which can lead to insufficient sample sizes for analysis (Karanth & Sunquist 1992; Varman & Sukumar 1995; Karanth et al. 2006,. Large mammalian carnivores may best exemplify these challenges (Karanth & Chellam 2009). Mammalian carnivores are among the most poorly understood higher order taxonomic groups in tropical forests (Johnson et al. 2009; Ramesh et al. 2012), and long-term monitoring efforts are lacking (Karanth et al. 2006). In contrast, detailed, long-term empirical investigations of large mammal population ecology, including carnivorestudies, have a longer history in open ecosystems, including tundra, alpine meadows, and grasslands (eg., Caughley 1967; Craighead et al. 1974; Sinclair et al. 1985; Ballard et al. 1997). As many carnivores are threatened with extinction (Karanth & Chellam 2009), increasing our knowledge of their population status and ecology may contribute to conservation planning efforts. The status of the largest felid of the western hemisphere, the jaguar (Panthera onca), is representative of an urgent conservation situation in the Americas (Weber & Rabinowitz 1996). Populations are declining rangewide (Caso et al. 2008), and the current distribution of the jaguar represents ~46% that of its early 20th century expanse (Sanderson et al. 2002). At the present, camera-trapping is the most widespread technique for monitoring jaguars, tigers, and other large tropical carnivores (eg., Karanth 1995; Silver et al. 2004; Silveira et al. 2009). This includes Neotropical pumas (Puma concolor) (Kelly et al. 2008; Negroes et al. 2010), regional populations of which are also declining across Latin America, particularly Brazil (Miotto et al. 2011; Mazzolli 2012). Although camera-trapping approaches have overcome many practical and logistical constraints associated with obtaining carnivore data in tropical forests, the recent, rapid evolution of noninvasive molecular techniques suggests their great potential in serving as an alternative. 38 Texas Tech University, Anthony J. Giordano, May 2015 Noninvasive genetic sampling (NGS) incorporating PCR-based approaches (cf. Mullis et al. 1986; White et al. 1989) and the extraction of DNA from sloughed intestinal cells on fecal material (cf. Albaugh et al. 1992) or ‘scats’, have demonstrated much promise for studying felids in tropical regions (Farrell et al. 2001; Mukherjee et al. 2010; Michalski et al. 2011). As with camera-trapping, NGS techniques have been applied in felid research across a variety of investigative contexts, including detecting presence (Palomares et al. 2002; Cossios & Angers 2006), determining minimum population size (Ernest et al. 2000; Miotto et al. 2007a), estimating abundance (Perez et al. 2006; Janecka et al. 2008; Sugimoto et al. 2012), and differentiating and monitoring sympatric species (Haag et al. 2009; Mukherjee et al. 2010; Michalski et al. 2011). Unlike camera-trapping however, the use of noninvasive molecular techniques can yield additional information, including unambiguous sex identification (Foran et al. 1997; Pilgrim et al. 2005), diet and predator-prey relationships (Farrell et al. 2001; Anwar et al. 2011), and genetic diversity, structure, and relatedness (Janecka et al. 2007; Haag et al. 2010; Janecka et al. 2011; Onorato et al. 2011; Miotto et al. 2012). Though NGS techniques are becoming increasingly cost-effective, their success still relies entirely on amplifying DNA from low quality and quantity sources (i.e., fecal material), so there remain limitations (Gerloff et al. 1995). Many factors can negatively impact amplification success rates and the capacity for species identification, including environmental conditions before collection (e.g., exposure to sun, water, etc.), differences in field and laboratory methods (e.g., collection and storage techniques, laboratory conditions and protocol, etc.), length of target DNA sequence fragments, and contamination (Murphy et al. 2002, 2003; Waits & Paetkau 2005; Broquet et al. 2007; Goossens & Salgado-Lynn 2013; Tende et al., in press). Therefore, while many primer sets may exist for different loci of a given taxon, their ability to perform consistently across all conditions can present a persistent obstacle to their more widespread use. Primers targeting the ATP-6 region of mammal mtDNA and optimized for identifying and discriminating between jaguar and puma scats (Haag et al. 2009) 39 Texas Tech University, Anthony J. Giordano, May 2015 recently proved effective when applied to ecological research of jaguars in the Atlantic Forest fragments (Haag et al. 2010). Though pumas are more widely-distributed than jaguars, local population declines and compromised habitat quality across parts of Latin America are of local conservation concern (Paviolo et al. 2009; Castilho et al. 2012). Moreover, sympatry with jaguars across much of the puma’s range, and interspecific competition for medium-large mammal prey (Farrell et al. 2001; Polisar et al. 2003; Scognamillo et al. 2003), makes the need to consistently and reliably differentiate their scats an important part of assessing their status. Because jaguar populations are declining in parts of the Gran Chaco (Altrichter et al. 2006; Quiroga et al. 2014), we wanted to test the efficacy of NGS for identifying, differentiating, and monitoring jaguars and pumas in the Paraguayan Chaco, as well as accurately confirming their sex. We also wanted to use NGS to assess the role of a large protected area in influencing the species and sex associated with felid samples in its proximity. Finally, we attempted to identify potentially important field and laboratory factors correlating with our success in identifying the species origin of samples. STUDY AREA The Gran Chaco occupies approximately 1.3 million km2 from southern Bolivia to south-central Argentina and is the largest forested biome in South America after the Amazon Basin (Bucher & Huszar 1999). All scat samples suspected to be of large felid origin were collected from the northern Paraguayan Chaco in the general vicinity of a 7,800 km2 protected area, Defenders of the Chaco National Park (DCNP), the largest protected area in the country (Figure 1). More than 60% of Paraguay’s approximately 400,000 km2 land area lies in the Gran Chaco ecoregion (Yahnke et al. 1998), an expansive, low-lying (< 500 m) semi-xeric forest and palm savanna alluvial plain extending west from just east of the Rio Paraguay (Bucher 1982; Redford et al. 1990). From the northwestern Chaco, average annual precipitation increases eastward and south from 500 mm (Boquerón) to approximately 1200 mm where the Rio Paraguay bisects the country (Sanchez 1973). Much of this is seasonal, though ‘dry seasons’ are capable of bearing both extreme flooding and prolonged drought. Mean annual temperatures range from 21 - 26 C° across northern 40 Texas Tech University, Anthony J. Giordano, May 2015 Paraguay, with marked seasonality and even extreme daily variation (Sanchez 1973). Temperatures of > 40°C are not uncommon from December – February, typically the country’s warmest months. MATERIALS & METHODS Scat Collection Scats of jaguars and pumas (Puma concolor) were collected both systematically and opportunistically in the northwestern Paraguayan Chaco. Although most were collected on roads and trails bordering and transecting DCNP, additional scats were collected on roads from other parts of the Gran Chaco Biosphere Reserve, including the adjacent Chaco Médanos (“Dunes of the Chaco”) National Park, unprotected areas along the frontier with Bolivia, the Chovereca – Agua Dulce region, and the Chaco-Pantanal transitional zone. We conducted all sampling between the months of April and November (2008-2010) when the Dry Chaco receives the least precipitation (Sanchez 1973) and is less likely to become inundated and inaccessible. We characterized scats as large felid in origin based on size, shape (e.g., segmentation, tapering), and type of prey remains visible (e.g., hair, bones, keratinous material); additional field sign (e.g., tracks, scrapings, prey remains) were also considered when present nearby. Scats not believed to originate from a large felid were removed from the road to avoid re-considering them during subsequent sampling. Apparent age and/or condition of a given scat was not a factor influencing the decision to collect scats. Scat samples were collected in their entirety, placed inside a new, gallon-size Ziploc ® plastic bag with silica gel beads and annotated with the date and location of collection. All bags were immediately stored in a dry, insulated cooler, and kept out of direct sunlight until such time that long-term storage at -20° C was possible; samples with high moisture content were repeatedly transferred to new bags until dry. Scat Storage and Processing After < 2 weeks, samples were stored for periods varying with field collection date at subfreezing temperatures (< 20° C) until they could be processed for DNA Extraction. Those portions of a scat containing sufficiently unbroken ‘surface’ or 41 Texas Tech University, Anthony J. Giordano, May 2015 mucus layer (i.e., intact, unexposed fecal material at scat’s surface) were sought for processing, as these were likely to yield the highest concentration of colonic epithelial cells (cf. Albaugh et al. 1992). This often included the tapered ends of scats, as well the scat ‘bottom’ (i.e., relative to the position encountered in the field), the latter of which was not exposed directly to sunlight. Portions where prey remains protruded through the top layer were avoided. After the best portions of a sample were identified, they were ‘cleaved’ with sterile razor blades, manipulated onto weighing paper with fine, sterile spatulas, and deposited into a 2.0 ml centrifuge tube. When possible, a minimum of three 2.0 ml tubes were each filled with at least 0.25 – 0.50 mg of dry fecal volume from a scat to ensure redundancy for downstream procedures. However as not all scat samples afforded the processing of this volume, as much fecal material as possible was taken from multiple parts of these samples and weighted. All tubes containing processed dry fecal material were kept at < 20°C until DNA extractions were performed. Species Identifications All scats were processed according to slight modifications of the DNA extraction procedure outlined in the Qiagen Stool Sample ® DNA Extraction Kit (#51504). We used primers developed by Haag et al. (2009) targeting a short segment of the mtDNA ATP synthase subunit 6 (ATP-6) gene for PCR amplification. All PCR’s occurred in a total reaction volume per sample of 15 μl containing 1.5 μl of PCR Buffer 10X (Qiagen), 0.6 μl of 25 mM MgCl2 (Qiagen), 0.3 μl of 10mM dNTPs (Qiagen), 0.08 μl of 7.5% BSA (Sigma-Aldrich), 0.3 μl of each primer at a concentration of 10 mM (DF2, DR1), 0.1 U of HotStarTaq ® (Qiagen), 5 μl of undiluted template DNA of unknown concentration, and 6.82 μl of distilled water. Amplifications were performed in PCR thermocycler machines (Mastercycler ®, Eppendorf, Westbury, NY). The reaction profile for each sample consisted of an initial denaturation of 5 minutes at 94°C, followed by 10 cycles (Touchdown) of (1) denaturation of 45 seconds at 94°C, (2) annealing at 60 - 51°C for 45 seconds (i.e., stepdown at - 1°C), and (3) extension at 72°C for 1.5 minutes, followed by 30 cycles of (4) denaturation at 94°C for 45 seconds, (5) annealing at 50°C for 45 seconds, and 42 Texas Tech University, Anthony J. Giordano, May 2015 (6) extension at 72°C for 1.5 minutes. A final extension step at 72°C for 10 minutes concluded the reaction where samples were held at 4°C. Post-PCR products were purified prior to sequencing by adding a pre-prepared 2 μl solution of Exonuclease I – Shrimp Alkaline Phosphate (ExoSAP). ExoSAP master mix (1x) total volume (2 μl) was prepared in advance and consisted of a total per sample reaction volume of: 0.37 μl of Exonuclease I (EXO), 0.18 μl of Shrimp Alkaline Phosphate (SAP), and 1.45 μl of distilled water. Post-PCR products received 2 μl of ExoSAP master mix to create a total volume of 17 μl per sample, which were then incubated in a PCR thermocycler machine (Mastercycler ®, Eppendorf, Westbury, NY, USA) for 15 minutes at 37°C, followed by an additional 15 minutes at 80°C; samples were then temporarily stored between -20°C and 4°C. Post-ExoSAP, all PCR products were submitted to the Genetics Core Facility of the University of Arizona (http://uagc.arl.arizona.edu) for sequencing in a 3730 Automated DNA Analyzer (Applied Biosystems, Foster City, CA, USA). Forward and reverse ATP-6 sequences successfully amplified from felid scat samples were scored and aligned using Sequencher ® (Gene Codes Corporation, Inc.) and compared to reference sequences in the National Center for Biotechnology Information (NCBI) GenBank database (Benson et al. 2005) with the partial match algorithm for program BLAST (Altschul et al. 1990). Final identification of puma or jaguar was based on match returns from the NCBI database with relatively high query (sequence) coverage, a high maximum identity (> ~90%), and a low E-value threshold (0.0). In the event sufficient separation of > 10% maximum identity was not possible, query coverage was suspect (<50 base pairs), and/or ranking was otherwise ambiguous, scat samples were subjected to re-extraction following the procedure elaborated above. Sex Identification To identify felid sex, we used amelogenin primers developed by Pilgrim et al. (2005) following slight modifications. PCR’s occurred in a total reaction volume per sample of 10 μl containing 1.0 μl of PCR Buffer 10X (Qiagen), 0.8 μl of 25 mM MgCl2 (Qiagen), 0.8 μl of 10 mM dNTPs (Qiagen), 0.05 μl of 7.5% BSA (Qiagen), 2 43 Texas Tech University, Anthony J. Giordano, May 2015 μl of each primer at a concentration of 5 mM (AMEL-F, AMEL-R), 0.2 μl of HotStarTaq ® (Qiagen), 3 μl of undiluted template DNA of unknown concentration, and 0.15 μl distilled water. All PCR’s were run with both positive (known jaguar) and negative controls in thermocyclers (Mastercycler ®, Eppendorf, Westbury, NY). To maximize HotStarTaq ® (Qiagen) effectiveness, each PCR profile began with 15 minutes at 95°C. The remainder of the profile consisted of 94°C at 5 minutes, followed by 45 cycles of (1) 94°C for 1 minute, (2) 51C° for 1 minute, and (3) 72°C for 30 seconds. PCR product (2 μl) for each sample was mixed with 2 μl of GelPilot 5x loading dye (Qiagen) and deposited into 2.5% agarose gels with 10 μl EtBr (Ethium Bromide). Gels were run at 100 V for 2 hours with 6 μl of 50 bp or 100 bp ladder per lane as gel standards and visualized under UV light after staining. Males were differentiated from females with a y-chromosome gene copy deletion, which yields double banding for the heterozygous condition (194 bp/ 214 bp); as females had no deletion (i.e., homozygous), they produced only a single band (214 bp). PCR products not manifesting bands after gelling at least twice were subject to repeated PCR attempts visualizing a volume of 5 μl on a new gel. To account for possible allelic dropout and avoid false positives, female identifications were confirmed only after three positive independent gel runs. Potential Factors Impacting Species Identification Success Overall success rates in identifying scat sample origin were recorded as the number of successes (i.e., positive ID’s) out of the total number of DNA extraction/ PCR attempts. Success in sexing jaguars and pumas was determined by the percentage of successful independent PCR attempts for confirmed large felids. We analyzed several factors possibly related to success in species identification. After subjecting eight laboratory technicians to the same training, we used success rates from our initial round of identifications as a proxy to assess human-introduced variability in the lab as a source of error. As technicians varied in the number of sample extractions each could perform, we tested for significant differences using chisquare [χ2] contingency and Fisher’s exact tests. 44 Texas Tech University, Anthony J. Giordano, May 2015 As samples were stored for varying periods before analysis, we tested the impact of long-term storage (i.e., time between collection and DNA extraction/ PCR; 1 month to 3 years) on species ID success with logistic regression analysis. Because moisture content and consistency caused the volume of individual samples to vary, we used logistic regression to also test for any relationship between initial dry sample mass (0.1 g – 1.0 g) and ability to ID species for all samples. In addition, as local environmental conditions (e.g., humidity, temperature, cloud cover, etc.) have the potential to impact laboratory success rates, we used eight discrete sampling events across two dry seasons in the dry Chaco as a proxy to examine the possible influence of environmental conditions on our ability to identify species origin. Finally, because DNA extraction and PCR amplification is a relatively costly process, we tested for differences in success rates between initial DNA extraction/ PCR and follow-up attempts to determine if re-extractions were effective and worthwhile. RESULTS We collected 554 scat samples believed to be of large felid origin across eight distinct field collection events in the Paraguayan Chaco between July 2008 and November 2010. Most (85.4%) were collected in the vicinity (< 5 km) of DCNP. We successfully identified species origin for 95.5% (n = 529) of all scat samples (Table 1). The majority were jaguar (54.0%), followed next by puma (34.3%), small felids (4.7%), and canids (2.0%). Approximately 5.1% of scats could not be identified to species after two independent attempts to extract due to poor DNA quality. Samples not jaguar or puma were not resolved further with additional means (i.e., generalized primers). However, among those small felids occurring at our study site receiving high initial match support were ocelot (Leopardus pardalis) and Geoffroy’s cat (L. geoffroyi); canid match identifications included foxes (Pseudolopex gymnocercus , Cerdocyon thous) and domestic dogs (Canis lupus familiaris). Positive controls consisting of mtDNA isolated from the blood of captive jaguars and pumas, as well as scats of known origin, confirmed the effectiveness of the PCR master mix and established a baseline for sample comparisons; all negative controls did not amplify. Of all samples, 41.2% (n=228) failed to initially yield a species identification and thus 45 Texas Tech University, Anthony J. Giordano, May 2015 were re-extracted. Of confirmed jaguar samples, 67.2% (n = 201) could be identified conclusively after the first effort to extract and amplify DNA (Table 1); the remaining jaguar samples (n = 98) required a second attempt before identification was successful. Of all puma samples, a comparable 67.9% (n = 129) were identified following the first extraction and amplification attempt (Table 1), with the remainder (n = 61) requiring a second attempt. New (second) DNA extractions were only initiated after two attempts at amplification (PCR) failed using the original dry volume. For each combined DNA extraction and PCR attempt, a logistic regression of both long-term storage time (i.e., interval between collection date and DNA extraction product; 6 months – 3 years) (U[R2]=0.0000, p=0.8768) and the initial dry volume (0.10 – 1 g) (U[R2]=0.0000, p=0.8673) failed to demonstrate a relationship between either predictor variable and on our ability to identify species. However, there appeared to be significant variation in technician performance (Table 2) as measured by individual success in yielding positive species identifications for all initial attempts (Likelihood Ratio χ2 = 75.478, p<0.0001, d.f. = 8; Pearson’s χ2 = 72.532, p<0.0001, d.f. = 8). Although we detected variation in species identification success among different collection events, these differences were very nearly significant at α = 0.05 (Likelihood Ratio χ2 = 14.019, p = 0.0508, d.f. = 7; Pearson’s χ2 = 13.969; p = 0.0517, d.f. = 7). Because the first 116 samples were initially placed inside non-sealable plastic and/or paper bags before being transferred to a 1-gallon re-sealable bag after < 14 days, we compared success rates between these samples and the remaining samples. Initial species identification rates were significantly lower for those samples not placed immediately into a re-sealable bag (48.3%) compared to those that were (65.4 %; Two-tailed Fisher’s Exact Test, p<0.001). However, ultimate species ID success rates for these samples (93.1%) were not significantly different from the entire sample. Overall, re-extraction and second PCR attempts were significantly more successful (87.3%; Two-tailed Fisher’s Exact Test, p < 0.0001) than initial efforts (61.9%). 46 Texas Tech University, Anthony J. Giordano, May 2015 We successfully confirmed the sex of 77.3% (n=231) of all jaguar samples (Table 2); male jaguars comprised the overwhelming majority (81.4%) of these samples. Only 18.6% (n=43) were identified as female. Following the first PCR amplification attempt, we successfully determined the sex for approximately 52.4% of these samples, with the remaining samples requiring additional effort (Table 3). Of all puma samples, we successfully identified the sex of 77.4% (n=147) of samples (Table 2); approximately 53.8 % (n=79) were male, and 46.3% were female (n=68). We were successful in identifying the sex of approximately 48.3% of puma samples on the first PCR amplification attempt, while the remaining samples required additional effort (Table 3). In those instances where new DNA extraction product was available due to initial failure in identifying species, this was used in subsequent PCR attempts of the amelogenin gene (Table 3). As part of regular sampling at or near (< 5 km) DCNP, we collected almost twice as many jaguar (n=278) as puma samples (n=147). The remaining scats (n=26) were from non-target species (i.e., small felids, canids). An additional 60 scats were collected opportunistically while making two additional trips to the Paraguay-Bolivia border in the far north dry Chaco: one close to Kaa-Iya Gran Chaco National Park, and another beginning west of Chaco Médanos National Park near another official border crossing with Bolivia. Although not part of a systematic sampling framework, we collected more than twice as many puma (n=37) samples as jaguar samples (n=15) at distances > 5 km from DCNP (Figure 1), as well as several small cat (n=6) and canid (n=2) scats. DISCUSSION The optimization of existing noninvasive genetic approaches and techniques can be challenging in new laboratory settings, while the development of new approaches can be time-consuming and costly. Lack of consensus approaches in the field can make evaluations of technique efficacy, as well as direct comparisons among studies, difficult or impossible. Although new techniques and approaches applicable to noninvasive field surveys are always emerging, we have observed few follow-up evaluations regarding the efficacy of any one approach. We chose to use the ATP-6 47 Texas Tech University, Anthony J. Giordano, May 2015 primers to identify large felid species based on their relatively short sequence lengths and high specificity for jaguars and pumas, their relative success in prior noninvasive contexts, and the assertion that the likelihood of amplifying DNA from prey remains was low (Haag et al. 2009, 2010). Efficacy of NGS Approach Some of our small felid samples (e.g., Leopardus) were not resolved further due to the lack of species reference sequences in the NCBI GenBank database, which has the potential to result in only a partial match, or explicitly lacks overlap with sample sequences (Naidu et al. 2012). Prey DNA amplification appeared to occur only once, when DNA from a lowland tapir (Tapirus terrestris) amplified with adequate sequence coverage within a jaguar scat sample. That our overall species identifications were so high can likely be attributed to collecting during the Dry Chaco winter, when precipitation was relatively scarce. Haag et al. (2009) report much lower overall success rates from two Atlantic Forest field sites (50-78% for), a biome of generally greater average annual humidity than the Chaco and thus one more likely to contribute to greater moisture retention and hydrolysis of DNA in scats. Furthermore, Haag et al. (2009) provide little detail on how scats were collected, mentioning only that a small portion (~6 g) of each was taken and stored in small vials at -20°C. We collected complete scats and sealed them in large, re-sealable plastic bags, most immediately upon collecting. Haag et al. (2009) do not elaborate on if multiple attempts to extract or amplify DNA were attempted, possibly trying only once. We also used HotStarTaq ® (Qiagen), potentially more effective at filtering out noise resulting from degraded DNA samples, for every PCR amplification attempt and all samples, possibly contributing to our higher success rates. In contrast, Haag et al. (2009) used both the analogous Platinum Taq ® (Invitrogen), as well as regular Taq Polymerase, for their PCR’s, and it is unclear what proportion of samples were subjected to each. Regardless, our study confirms the efficacy of these primers for monitoring and differentiating jaguar and puma scats in the field. Successful sex identification was comparably lower than that for species identification for all samples. This is unsurprising however, as mtDNA amplification 48 Texas Tech University, Anthony J. Giordano, May 2015 rates are generally higher than those for nuclear DNA in a noninvasive context (Waits & Paetkau 2005). However, the convenience of determining sex without sequencing PCR product made processing convenient and saved considerable time and expense. We believe we were relatively successful (~77%) in confirming sex for samples overall, as the lack of the need to sequence product permitted us to repeatedly run gels in the event of initial sample failure. It is possible that variation in gel preparation component over time may have resulted in unclear reads for some attempts, which we indicated as failure. This is an important consideration, as small variations in DNA preservation, extraction, and PCR protocol might lead to varying performance results in the laboratory (Waits & Paetkau 2005; Beja-Pereira et al. 2009). Overall and first attempt identification rates were similar for both jaguars and pumas, suggesting that ATP-6 primers performed comparably well for both species as with Haag et al. (2009). Interestingly, neither initial dry sample volume, nor longterm storage at -20°C, had any effect on final species identification rates overall. Although it appears few studies have examined the effects of dry volume, Wehausen et al. (2004) claimed that DNA amplification was generally not sensitive to the volume of dry fecal material used. Although we saw no evidence of a decline in amplification success, even after several years for some samples, other studies have concluded that long intervals of sample storage (e.g., > 3-6 months) for noninvasivelycollected samples had a negative impact on DNA amplification success (Frantzen et al. 1998; Murphy et al. 2002; Roon et al. 2003). Possible recent refinement of fecal DNA extraction and PCR protocols, and our use of optimized ATP-6 mtDNA primers and HotStarTaq ® (Qiagen, Inc.), may have mitigated the impact of long-term storage. Primer optimization has also improved success rates for nuclear DNA in previous studies (Lampa et al. 2008). As we did not also use more generalized primers (e.g., Verma & Singh 2003; Naidu et al. 2012), strict comparisons are not possible. Despite relatively high success rates in identifying the species origin of samples, including those subjected to long-term storage (i.e., 1-3 years), our study suggests that human-introduced variability, in our case among laboratory technicians, might be a contributing role to differences in identification success. This is true even 49 Texas Tech University, Anthony J. Giordano, May 2015 when exposed to the same standardized laboratory protocol. Although this variability meant little to our overall success rates (i.e., due to re-extraction efforts), it could have implications for similar studies. Waits and Paetkau (2005) and Beja-Pereira et al. (2009) in their detailed reviews of factors influencing NGS did not address humanintroduced variation, and we did not find a study discussing it in detail. Inherent variability among individuals might exist in how scats are initially stored and collected, prepared for PCR, gels are prepared, and the scat portions chosen for processing. Variation in the latter for example can have important implications with respect to overall laboratory success rates (Wehausen et al. 2004; Stenglein et al. 2010), suggesting that per sample DNA quality can vary greatly and be more important than dry volume. This is not inconsistent with our findings and could even be a factor contributing to differences in success rates between first and second DNA extraction attempts, as not all samples possessed regions obviously identifiable surface or mucus layer (i.e., areas were colonic epithelial cells might be expected to occur). In addition to representing a different portion of the scat, re-extracted DNA elution was also subjected to a shorter storage time (i.e., < 3 weeks) in the freezer (20°C) before being amplified, possibly contributing to our higher second attempt success rates. It is reasonable that longer latency intervals might negatively impact success, though prior reviews of NGS (e.g., Waits & Paetkau 2005; Beja-Pereira et al. 2009) have not explicitly addressed this. During initial DNA extraction attempts, this interval varied extensively in adherence to a predefined, cost-effective sample processing protocol. We should however also emphasize this interval might confound the validity of interpretations regarding human-introduced error, as we believe it varied considerably among individual technicians processing samples in batches. Our findings are also consistent with the importance of field environmental conditions (e.g., sunlight prevalence or intensity, surface temperature, humidity, sample moisture, etc.) as a factor in contributing to species confirmation. Our analysis of success in species identification among scat collection events was almost significant (p~0.051), suggesting that initial conditions may have played a role. Such factors have already been demonstrated to negatively impact DNA quality in noninvasively 50 Texas Tech University, Anthony J. Giordano, May 2015 collected scat samples under both free-ranging and captive conditions (Frantzen et al. 1998; Waits & Paetkau 2005; Beja-Pereira et al. 2009; Goossens & Salgado-Lynn 2013; Tende et al., in press). Haag et al. (2009) for example achieved different amplification success rates for two different localities, despite both being collected in different parts of the same general Atlantic Forest biome. Finally, it is possible that our methods for storing and preserving samples contributed to high species identification success rates. As we made no effort to vary from the use of large re-sealable plastic bags at -20°C for all samples, it is not possible to assess this with confidence. Other studies have however found great variability in the effectiveness of methods to preserve and store samples for processing (Frantzen et al. 1998; Murphy et al. 2000, 2002; Nsubuga et al. 2004). Wasser et al. (1997) concluded that silica-based drying and preservation using sealable freezer bags proved the most practical and superior approach. In contrast, Murphy et al. (2002) later determined that methods relying on either ethanol or DMSO buffer solution for preservation were the more effective, while Frantzen et al. (1998) found no significant differences in mtDNA amplification success among different preservation methods. Storing samples in paper and non-sealable plastic bags at -20°C until samples were processed, Naidu et al. (2011) and Rinkevich (2012) met with comparably lower species identification rates (65% and 47% respectively) for felid and canid scats respectively collected from the arid southwestern United States. However, both also relied on generalized mtDNA cytochrome b primers (Verma & Singh 2003), which target longer nucleotide sequences than those used in this study (i.e., ~470 bp vs. ~170 bp). Jaguar and Puma Samples In our efforts to identify all possible large felid scats at DCNP, we ultimately collected twice as many jaguar scats as puma. The proportionally larger sample of jaguar scats may be due to differences in population density between the two species at the protected area. Despite the potential for correlation between detection rates (e.g., scat encounter rates) and density (Carbone et al. 2001), the former do not 51 Texas Tech University, Anthony J. Giordano, May 2015 necessarily always lead to reliable density estimates, even when performed in the context of a systematic sampling effort (Jennelle et al. 2002; MacKenzie et al. 2002, 2006). Still, Stander (1998) found that the use of sign counts along roads showed a strong linear relationship with the density of three large African carnivores, including leopards. In addition, Jhala et al. (2011) confirmed through the use of mark-recapture models that tiger abundance could be reasonably approximated across a variety of habitats and a range of tiger densities by quantifying the presence of sign. Alternatively, the proportional differences in samples from each species may be due to interspecific differences in behavior and activity patterns, in this case when anthropogenic impacts on the landscape are less severe. At one site in Belize for example, jaguars were photo-trapped ten timesmore frequently than pumas over a single 3-month period (Davis et al. 2011). In the Bolivian Chaco-Chiquitana forests, an area very close geographically to DCNP, estimates of puma densities (3.7/100 km2) were slightly higher than that for jaguars (2.2 – 3.3/100 km2) for the same general region (Maffei et al. 2004; Kelly et al. 2008), consistent with the idea that activity may be important in our study area. Jaguars may be less reluctant to use roads and trails than pumas and travel on them for greater distances irrespective of the local abundance of either predator. This might be particularly true in closer proximity to a protected area, where fewer anthropogenic impacts may favor increased jaguar activity or abundance. Evidence exists that although pumas may also benefit from protection (Paviolo et al. 2009), jaguars may be comparably more vulnerable to anthropogenic impacts on the surrounding landscape, including forest fragmentation, direct persecution, and poaching of prey (Paviolo et al. 2008; Haag et al. 2010; De Angelo et al. 2011). Proximity to a protected area may also impact the competitive dynamics between jaguars and pumas. Jaguars may be more likely to opportunistically kill pumas under these conditions, and interspecific killing of this sort is not unusual among carnivore communities (Palomares & Caro 1999; Linell & Strand 2000). Despite strong spatial overlap, Romero-Munoz et al. (2010) and Harmsen et al. (2009) found clear temporal segregation between individual jaguars and pumas in the 52 Texas Tech University, Anthony J. Giordano, May 2015 Bolivian Chaco and Belize respectively, a finding consistent with avoidance of jaguars by pumas. At our study site, pumas might be less likely to use or travel as far along roads in areas when jaguars are present, and thus fewer puma scats might be expected. Such roads may act in part as trails do in other geographical regions due to their unpaved nature, relatively low traffic volume, proximity to the protected area, and remote location. Interestingly however, a study in Belize concluded that pumas on average used trails more completely and over greater distances than jaguars (Harmsen et al. 2010). Another possibility that cannot be discounted is that there was some bias in the size or morphology of samples collected. For example, we may have unwittingly not collected scats originating from pumas, which could have been assessed in the field as originating from a non-target species. This may have led to a preconception of what a ‘large felid’ scat might look like, particularly if no additional sign was present. Scats of sympatric carnivores can demonstrate considerable overlap in overall size and morphology, and thus consistent differentiation is generally not possible (Foran et al. 1997; Farrell et al. 2001; Davison et al. 2002). This can be true even with the use of scat detection dogs (Rinkevich 2012), which were not logistically feasible or costeffective for us to use over the area we sampled. That we collected several dozen scats of non-target species (i.e., small cats, canids) suggested some overlap and/or contextual bias may have occurred during sampling. Similarly, we collected a few relatively small scats (n=5) with the belief they originated from small felids, which proved to be puma in origin. Miotto et al. (2007b) noted a similar problem for puma and ocelots scats over a much smaller sample. Of the smaller sample of scats we collected opportunistically elsewhere in the northern dry Chaco (i.e., to the west and north of the DCNP), we collected twice as many puma scats as jaguar scats. Most were collected along rural roads similar to those bordering or transecting the park; however, these roads were flanked by private land on both sides, never bordering protected land. Of those samples collected closest to the Paraguay-Bolivia border (<20 km), all were identified as puma. Although our sample size for these opportunistic collections was much smaller than for systematic 53 Texas Tech University, Anthony J. Giordano, May 2015 collections at DCNP (n=449 vs. n=60), our findings are consistent with puma activity patterns exhibiting greater resilience than jaguars amidst anthropogenic landscape modifications (Paviolo et al. 2008, 2009; Lyra-Jorge et al. 2008; Mazzolli 2010; De Angelo et al. 2011). Among those scats sampled systematically at the Park, less than 1 in 5 jaguar scats collected were from a female. In contrast, male and female puma scats were collected in nearly equal proportions. It is unclear why this disparity existed at Paraguay’s largest protected area, a forest region that is contiguous with the most expansive and intact in the Dry Chaco of Paraguay. Palomares et al. (2012) reported on the consistent male-bias in scat samples collected off roads and trails in the field across multiple sites; in contrast, samples for pumas or ocelots varied more widely in sex ratio for these same sites. However, here we report this disparity for a relatively large sample of confirmed jaguar scats collected widely across a single large, protected study area. Female jaguars in our study area may be more secretive and less likely to use roads and trails; across the jaguar’s range, females appear to be consistently recorded much less than males via camera-trap (Maffei et al. 2011). This might be because males range comparably more widely in their movements, while females exhibit more fidelity to a core territory. In Belize, female activity patterns were also more sensitive to areas of human disturbance (Foster et al. 2010). Conde et al. (2010) noted that female jaguars in Mexico also responded negatively to human disturbance, avoiding roads and using available habitat in different proportions than males. In our study area however, traffic along most of the roads (all unpaved) we sampledcan be characterized as seasonally intermittent, and we infrequently encountered other vehicles during our sampling events. Alternatively, the sex ratio of jaguars may actually be skewed at DCNP, possibly indicative of a declining population. If true, this would be of great conservation concern, as this park represents the largest contiguous expanse of Dry Chaco in all of Paraguay. CONCLUSIONS Using mtDNA primers optimized for pumas and jaguars, we achieved some of the highest reported species identification rates in a noninvasive genetic field sampling 54 Texas Tech University, Anthony J. Giordano, May 2015 context. We also report on the successful application of amelogenin primers in the sex identification of large felid scat samples. We believe the specificity of primers, their optimization for use on felids, their relatively short target sequence lengths, and the possible efficacy of our collection and storage methods in preventing DNA oxidation, contributed to our overall success rates. Although human-introduced error may have impacted our ability to successfully identify scats initially, short time intervals between DNA extraction procedures and PCR amplification may have increased our identification success rates for sample re-extractions. Lack of consistency in the portions of scats taken for processing may have been a factor in the failure of samples to yield an ID, though this is unclear. Moreover, dry sample volume, and time between collection and DNA extractionin storage at < -20 C°, were not important predictors of our ability to successfully ID samples. Overall, our results are consistent with the idea that environmental context and conditions in which scats are deposited, including the general aridity of our region during sampling events, may have played a role in our high overall success rates, and we note the great potential to recover and amplify viable mtDNA with optimized, short fragment sequence primers even after scats have been stored for more than a year. Although we can confirm the efficacy of primers and recommend their use in noninvasive jaguar and puma monitoring, we caveat that sample identification success rates will probably vary considerably across geographical locations due to differences in local environmental conditions, as well as varying laboratory conditions and reagents. We therefore recommend a pilot field study to evaluate success rates in species and sex identification of scat samples collected from a given study area, and optimization for analysis given a particular suite of laboratory conditions, before commencing a more thorough investigation and a greater investment of resources. We also recommend additional effort when confirming the origin of noninvasive samples should initial attempts fail if time and resources permit. Finally, we believe the extremely low number of confirmed female jaguars in a large protected area, particularly when compared directly to pumas for the same sampling effort, either reflects a sampling bias when using this method, or may be at least partially 55 Texas Tech University, Anthony J. Giordano, May 2015 representative of skewed sex ratios in the northern Paraguayan Chaco. Similarly, that puma samples were predominant short distances from the protected area is consistent with the findings of prior research that landscape transformation may have a differentially negative impact on jaguars compared to pumas. Future investigations should attempt to address more conclusively the status of females, as well as if activity patterns and road use might account for differences between jaguars and pumas. 56 Texas Tech University, Anthony J. Giordano, May 2015 REFERENCES Albaugh GP, Iyengar V, Lohani A, Malayeri M, Bala S, Nair PP (1992). Isolation of exfoliated colonic epithelial cells, a novel non-invasive approach to the study of cellular markers. International Journal of Cancer 52, 347-50. Altrichter M, Boaglio G, Perovic P (2006). The decline of jaguars (Panthera onca) in the Argentine Chaco. Oryx40, 302-9. Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990). Basic local alignment search tool. Journal of Molecular Biology215, 403-10. Anwar MB, Jackson R, Nadeem MS, Janecka JE, Hussain S, Beg MA, Muhammad G, Qayyum M (2011). Food habits of the snow leopard Panthera uncia (Schreber, 1775) in Balistan, Northern Pakistan. European Journal of Wildlife Research57, 1077-83. Ballard WB, Ayres LA, Krausman PR, Reed DJ, Fancy SG (1997). Ecology of wolves in relation to a migratory caribou herd in northwest Alaska. Wildlife Monographs135, 1-47. Beja-Pereira A, Oliveira R, Alves P, Schwartz MK, Luikart G (2009). Advancing ecological understandings through technological transformations in noninvasive genetics. Molecular Ecology Resources9, 1279-1301. Benson DA, Karsh-Mizrachi I, Lipman DJ, Ostell J, Wheeler DL (2005). GenBank. Nucleic Acids Research33, D34-D38. Broquet T, Menard N, Petit E (2007). Noninvasive population genetics: a review of sample source, diet, fragment length and microsatellite motif effects on amplification success and genotyping error rates. Conservation Genetics8, 249-60. Bucher EH (1982). Chaco and caatinga: South American arid savannas, woodlands, and thickets. In: Huntley B, Walker B, eds. Ecology of Tropical Savannas. Springer Press, New York, NY, pp. 48-79. Bucher EH, Huszar PC (1999). Sustainable management of the Gran Chaco of South America: ecological promise and economic constraints. Journal of Environmental Management57, 99-108. 57 Texas Tech University, Anthony J. Giordano, May 2015 Carbone C, Christie S, Conforti K, Coulson T, Franklin N, Ginsberg JR, Griffiths M, Holden J, Kawanashi K, Kinnaird M, Laidlaw R, Lynam A, Macdonald DW, Martyr D, McDougal C, Nath L, O’Brien T, Seidensticker J, Smith DJL, Sunquist M, Tilson R, Wan Shahruddin WN (2001). The use of photographic rates to estimate densities of tigers and other cryptic mammals. Animal Conservation 4, 75-79. Caso A, Lopez-Gonazlez C, Payan E, Eizirik E, Oliveira TG, Leite-Pitman R, Kelly M, Valderrama C (2008). Panthera onca. In: IUCN 2013. IUCN Red List of Threatened Species. Version 2013.2. [Cited 15 October 2013.] Available from URL: http://www.iucnredlist.org/. Castilho CS, Marins-Sa LG, Benedet RC, Freitas TRO (2012). Genetic structure and conservation of mountain lions in the South-Brazilian Atlantic Rain Forest. Genetics and Molecular Biology35, 65-73. Caughley G (1967). Calculation of population mortality rate and life expectancy for thar and kangaroos from the ratio of juveniles to adults. New Zealand Journal of Science 10, 578-84. Conde, DA, Colchero F, Zarza H, Christensen Jr NL, Sexton JO, Manterola C, Chavez C, Rivera A, Azuara D, Ceballos G (2010). Sex matters: modeling male and female habitat differences for jaguar conservation. Biological Conservation143, 1980-88. Cossios D, Angers B (2006). Identification of Andean felid feces using PCR-RFLP. Mastozoologia Neotropical13, 239-44. Craighead JJ, Varney JR, Craighead Jr FC (1974). A population analysis of the Yellowstone grizzly bears; Montana Cooperative Wildlife Research Unit Bulletin No. 40. University of Montana Press, Missoula, MT. Davis ML, Kelly MJ, Stauffer DF (2011). Carnivore co-existence and habitat use in the Mountain Pine Ridge Forest Reserve, Belize. Animal Conservation14, 5665. 58 Texas Tech University, Anthony J. Giordano, May 2015 Davison A, Birks JDS, Brookes RC, Braithwaite TC, Messenger JE. 2002. On the origin of faeces: morphological versus molecular methods for surveying rate carnivores from their scats. Journal of Zoology 257, 141-43. De Angelo C, Paviolo A, Di Bitetti M (2011). Differential impact of landscape transformation on pumas (Puma concolor) and jaguars (Panthera onca) in the Upper Parana Atlantic Forest. Diversity and Distributions17, 422-36. Ernest HB, Penedo MC, May BP, Syvanen M, Boyce WM (2000). Molecular tracking of mountain lions in the Yosemite valley region in California: genetic analysis using microsatellites and faecal DNA. Molecular Ecology9, 433-41. Farrell LE, Roman J, Sunquist ME (2001). Dietary separation of sympatric carnivores by molecular analysis of scats. Molecular Ecology9, 1583-90. Foran DR, Crooks KR, Minta SC (1997). Species identification from scat: an unambiguous genetic method. Wildlife Society Bulletin25, 835-39. Foster RJ, Harmsen BJ, Doncaster CP (2010). Habitat use by sympatric jaguars and pumas across a gradient of human disturbance. Biotropica42, 724-31. Frantzen MAJ, Silk JB, Ferguson JW, Wayne RK, Kohn MH (1998). Empirical evaluation of preservation methods for fecal DNA. Molecular Ecology7, 142328. Gerloff U, Scholtterer C, Rassmann K, Rambold I, Hohmann G, Fruth B, Tautz D (1995). Amplification of hypervariable simple sequence repeats (microsatellites) from excremental DNA of wild living bonobos (Pan niscus). Molecular Ecology4, 515-18. Goossens B, Salgado-Lynn M (2013). Advances and difficulties of molecular tools for carnivore conservation in the tropics. Raffles Bulletin of ZoologyS28, 43-53. Haag T, Santos AS, De Angelo C, Srbek-Araujo AC, Sana DA, Morato RG, Salzana FM, Eizirik E (2009). Development and testing of an optimized method for DNA-based identification of jaguar (Panthera onca) and puma (Puma concolor) faecal samples for use in ecological and genetic studies. Genetica136, 505-12. 59 Texas Tech University, Anthony J. Giordano, May 2015 Haag T, Santos AS, Sana DA, Morato RG, Cullen Jr L, Crawshaw Jr PG, De Angelo C, Di Bitetti MS, Salzano FM, Eizirik E (2010). The effect of habitat fragmentation on the genetic structure of a top predator: loss of diversity and high differentiation among remnant populations of Atlantic Forest jaguars. Molecular Ecology19, 4906-21. Harmsen BJ, Foster RJ, Silver SC, Ostro LET, Patrick Doncaster C (2009). Spatial and temporal interactions of sympatric jaguars (Panthera onca) and pumas (Puma concolor) in a Neotropical forest. Journal of Mammalogy90, 612-20. Harmsen BJ, Foster RJ, Silver S, Ostro L, Doncaster CP (2010). Differential use of trails by forest mammals and the implications for camera-trap studies: a case study from Belize. Biotropica42, 126-33. Janecka JE, Blankenship TL, Hirth DH, Tewes ME, Kilpatrick CW, Grassman LI (2007). Evidence for male-biased dispersal in bobcats Lynx rufus using relatedness analysis. Wildlife Biology13, 38-47. Janecka JE, Jackson R, Yuquang Z, Diqiang L, Munkhstog B, Buckley-Beason V, Murphy WJ (2008). Population monitoring of snow leopards using noninvasive collection of scat samples: a pilot study. Animal Conservation11, 401-11. Janecka JE, Munkhtsog B, Jackson R, Naranbaatar G, Mallon D, Murphy W (2011). Comparison of noninvasive genetic and camera-trapping techniques for surveying snow leopards. Journal of Mammalogy92, 771-83. Jennelle CS, Runge MC, MacKenzie DI (2002). The use of photographic rates to estimate densities of tigers and other cryptic mammals: a comment on misleading conclusions. Animal Conservation5, 119-20. Jhala Y, Qureshi Q, Gopal R (2011). Can the abundance of tigers be assessed from their signs? Journal of Applied Ecology48, 14-24. Johnson A, Vongkhamheng C, Saithongdam T (2009). The diversity, status, and conservation of small carnivores in a montane tropical forest in northern Laos. Oryx43, 626-33. 60 Texas Tech University, Anthony J. Giordano, May 2015 Karanth KU (1995). Estimating tiger Panthera tigris populations populations from camera-trap data using capture-recapture models. Biological Conservation 71, 333-38. Karanth KU, Sunquist ME (1992). Population structure, density, and biomass of large herbivores in the tropical forests of Nagarahole, India. Journal of Tropical Ecology8, 21-35. Karanth KU, Chellam R (2009). Carnivore conservation at the crossroads. Oryx43, 12. Karanth KU, Nichols JD, Kumar NS, Hines JE (2006). Assessing tiger population dynamics using photographic capture-recapture sampling. Ecology87, 2925-37. Kelly MJ, Noss AJ, Di Bitetti MS, Maffei L, Arispe RL, Paviolo A, De Angelo CD, Di Blanco YE (2008). Estimating puma densities from camera trapping across three study sites: Bolivia, Argentina, and Belize. Journal of Mammalogy 89, 408-18. Lampa S, Gruber B, Henle K, Hoehn M (2008). An optimisation approach to increase DNA amplification success of otter faeces. Conservation Genetics9, 201-10. Linnell JD, Strand O (2000). Interference interactions, co-existence, and conservation of mammalian carnivores. Diversity & Distributions6, 169-176. Lyra-Jorge MC, Ciocheti G, Pivello VR (2008). Carnivore mammals in a fragmented landscape in northeast of Sao Paulo State, Brazil. Biodiversity Conservation17, 1573-80. MacKenzie DI, Nichols JD, Lacham GB, Droege S, Royle JA, Langtimm CA (2002). Estimating site occupancy rates when detection probabilities are less than one. Ecology83, 2248-55. MacKenzie DI, Nichols JD, Royle JA, KH Pollock, Bailey LL, Hines JE (2006). Occupancy estimation and modeling: inferring patterns and dynamics of species occurrence. Academic Press, Burlington, MA. Maffei L, Cuellar E, Noss A (2004). One thousand jaguars in Bolivia’s Chaco? Camera trapping in the Kaa-Iya National Park. Journal of Zoology262, 295304. 61 Texas Tech University, Anthony J. Giordano, May 2015 Maffei L, AJ Noss, SC Silver, MJ Kelly (2011). Abundance/density case study: jaguars in the Americas. In: O’Connell AF, Nichols JD, Karanth KU, eds. Camera traps in animal ecology: methods and analyses. Springer Press, New York, pp. 119-44. Mazzolli M (2010). Mosaics of exotic forest plantations and native forests as habitats of pumas. Environmental Management46, 237-53. Mazzolli M (2012). Natural recolonization and suburban presence of pumas (Puma concolor) in Brazil. Journal of Ecology and the Natural Environment14, 34462. Michalski F, Valdez FP, Norris D, Zieminski C, Kashivakura CK, Trinca C, Smith HB, Vynne C, Wasser SK, Metzger JP, Eizirik E. 2011. Successful carnivore identification with faecal DNA across a fragmented Amazonian landscape. Molecular Ecology Resources11, 862-71. Miotto RA, Rodrigues FP, Ciocheti G, Galetti Jr PM (2007a). Determination of the minimum population size of pumas (Puma concolor) through fecal DNA analysis in two protected cerrado areas in the Brazilian southeast. Biotropica39, 647-54. Miotto RA, Ciocheti G, Rodrigues FP, Galetti Jr PM (2007b). Identification of pumas (Puma concolor (Linnaeus, 1771) through faeces: a comparison between morphological and molecular methods. Brazilian Journal of Biology67(4S), 963-65. Miotto RA, Cervini M, Figueiredo MG, Begotti RA, Galetti Jr PM (2011). Genetic diversity and population structure of pumas (Puma concolor) in southeastern Brazil: implications for conservation in a human-dominated landscape. Conservation Genetics 12, 1447-55. Miotto RA, Cervini M, Begotti RA, and Galetti Jr PM (2012). Monitoring a puma (Puma concolor) population in a fragmented landscape in southeast Brazil. Biotropica44, 98-104. 62 Texas Tech University, Anthony J. Giordano, May 2015 Mullis K, Faloona F, Scharf S, Saiki R, Horn G, Erlich H (1986). Specific enzymatic amplification of DNA in vitro: the polymerase chain reaction. Cold Spring Harbor Symposia on Quantitative Biology51, 263-73. Mukherjee S, Ashalakshmi C, Home C, Ramakrishnan U (2010). An evaluation of the PCR-RFLP techinique to aid molecular-based monitoring of felids and canids in India. BMC Research Notes3, 159. http://www.biomedcentral.com/17560500/3/159. Murphy MA, Waits LP, Kendall KC (2000). Quantitative evaluation of fecal drying methods for brown bear DNA analysis. Wildlife Society Bulletin28, 951-57. Murphy MA, Waits LP, Kendall KC, Wasser SK, Higbee JA, Bogden R (2002). An evaluation of long-term preservation of brown bear (Ursus arctos) faecal DNA samples. Conservation Genetics3, 435-40. Murphy MA, Waits LP, Kendall KC (2003). The influence of diet on faecal DNA amplification and sex identification in brown bears (Ursus arctos). Molecular Ecology12, 2261-65. Naidu A, Smythe LA, Thompson RW, Culver M (2011). Genetic analysis of scats reveals minimum number and sex of recently document mountain lions. Journal of Fish and Wildlife2, 106-11. Naidu A, Fitak RR, Munguia-Veja A, Culver M (2012). Novel primers for complete mitochondrial cytochrome b gene sequencing in mammals. Molecular Ecology Resources12, 191-96. Negroes N, Sarmento P, Cruz J, Eira C, Revilla E, Fonseca C, Sollmann R, Torres NM, Furtado MM, Jacomo ATA, Silveira L (2010). Use of câmera-trapping to estimate puma density and influencing factors in Central Brazil. Journal of Wildlife Management 74, 1195-1203. Nsubuga AM, Robbins MM, Roeder AD, Morin PA, Boesch C, Vigilant L. 2004. Factors affecting the amount of genomic DNA extracted from ape faeces and the identification of an improved sample storage method. Molecular Ecology13, 2089-94. 63 Texas Tech University, Anthony J. Giordano, May 2015 O’Connell AF, Nichols JD, Karanth KU (eds). 2011. Camera traps in animal ecology. Springer Publishing, Tokyo, Japan. Onorato D, Desimone R, White C, Waits LP (2011). Genetic assessment of paternity and relatedness in a managed population of cougars. Journal of Wildlife Management75, 378-84. Palomares F, Caro TM (1999). Interspecific killing among mammalian carnivores. The American Naturalist153, 492-508. Palomares F, Godoy JA, Piriz A, O’Brien SJ, Johnson WE (2002). Faecal genetic analysis to determine the presence and distribution of elusive carnivores: design and feasibility for the Iberian lynx. Molecular Ecology11, 2171-82. Palomares F, Roques S, Chavez C, Silveira L, Keller C, Sollmann R, do Prado DM, Torres PC, Adrados B, Godoy JA, Jacomo ATA, Torres NM, Furtado MM, Lopez-Bao JV (2012). High proportion of male faeces in jaguar populations. PLoS ONE7(12), e52923. DOI:10.1371/journal.pone.0052923. Paviolo A, De Angelo CD, Di Blanco YE, Di Bitetti MS (2008). Jaguar Panthera onca population decline in the Upper Parana Atlantic Forest of Argentina and Brazil. Oryx42, 554-61. Paviolo A, Di Blanco YE, De Angelo CD, Di Bitetti MS (2009). Protection affects the abundance and activity patterns of pumas in the Atlantic Forest. Journal of Mammalogy90, 926-34. Perez I, Geffen E, Mokady O (2006). Critically endangered Arabian leopards Panthera pardus nimr in Israel: estimating population parameters using molecular ecology. Oryx 40, 295-301. Pilgrim KL, McKelvey KS, Riddle AE, Schwartz MK (2005). Felid sex identification based on noninvasive genetic samples. Molecular Ecology Notes 5, 60-61. Polisar J, Maxit I, Scognamillo D, Farrell L, Sunquist ME, and Eisenberg JF (2003). Jaguars, pumas, their prey base, and cattle ranching: ecological interpretations of a management problem. Biological Conservation109, 297-310. 64 Texas Tech University, Anthony J. Giordano, May 2015 Quiroga VA, Boaglio GI, Noss AJ, Di Bitetti MS (2014). Critical population status of the jaguar (Panthera onca) in the Argentina Chaco: camera-trap surveys suggest recent collapse and imminent regional extinction. Oryx 48, 141-48. Ramesh T, Sridharan N, Sankar K, Quereshi A, Selvan KM, Gokulakkannan N, Francis P, Narasimmarajan K, Jhala YV, Gopal R (2012). Status of large carnivores and their prey in tropical rainforests of south-western Ghats, India. Tropical Ecology53, 137-48. Redford KH, Taber A, Simonetti JA (1990). There is more to biodiversity than the tropical rain forests. Conservation Biology4, 328-30. Rinkevich SE (2012). An assessment of abundance, diet, and cultural significance of Mexican gray wolves in Arizona(PhD dissertation). University of Arizona, Tucson, AZ. Romero-Munoz A, Maffei L, Cuellar E, Noss AJ (2010). Temporal separation between jaguar and puma in the dry forests of southern Bolivia. Journal of Tropical Ecology26, 303-11. Roon DA, Waits LP, Kendall KC (2003). A quantitative evaluation of two methods for preserving hair samples. Molecular Ecology Notes3, 163-66. Sanchez TF (1973). The climate of Paraguay. In: Gorham JR, ed. Paraguay Ecological Essays. Academy of the Arts and Sciences of the Americas, Miami, Florida, pp. 33-38. Sanderson EW, Redford KH, Chetkiewicz C-LB, Medellin RA, Rabinowitz AR, Robinson JG, and Taber AB (2002). Planning to save a species: the jaguar as a model. Conservation Biology16, 58-72. Scognamillo D, Maxit IE, Sunquist M, Polisar J (2003). Coexistence of jaguar (Panthera onca) and puma (Puma concolor) in a mosaic landscape in the Venezuelan llanos. Journal of Zoology259, 269-79. Silveira L, Jacomo ATA, Astete S, Sollmann R, Torres NM, Furtado MM, MarinhoFilho J (2009). Density of the Near Threatened jaguar Panthera onca in the caatinga of north-eastern Brazil. Oryx44, 104-09. 65 Texas Tech University, Anthony J. Giordano, May 2015 Silver SC, Ostro LET, Marsh LK, Maffei L, Noss A, Kelly MJ, Wallace RB, Gomez H, Crespo GA (2004). The use of camera traps for estimating jaguar (Panthera onca) abundance and density using capture/recapture analysis. Oryx38,148-54. Sinclair ARE, Dublin H, Borner H (1985). Population regulation of Serengeti Wildebeest: a test of the food hypothesis. Oecologia65, 266-68. Stander PE (1998). Spoor counts as indices of large carnivore populations: the relationship between spoor frequency, sampling effort, and true density. Journal of Applied Ecology35, 378-85. Stenglein JL, De Barba M, Ausband DE, Waits LP (2010). Impacts of sampling location within a faeces on DNA quality in two carnivore species. Molecular Ecology Resources10, 109-14. Sugimoto T, Nagata J, Aramilev VV, McCullough DR (2012). Population size estimation of Amur tigers in Russian Far East using noninvasive genetic samples. Journal of Mammalogy93, 93-101. Tende T, Hansson B, Ottosson U, Bensch S (2014). Evaluating preservation medium for the storage of DNA in African lion Pantheraleo faecal samples. Current Zoology, 60, 351-358. Thompson WL, ed (2004). Sampling rare or elusive species: concepts, designs, and techniques for estimating population parameters. Island Press, Washington, DC. Varman KS, Sukumar R (1995). The line transect method for estimating densities of large mammals in a tropical deciduous forest: an evaluation of models and field experiments. Journal of Bioscience20, 273-87. Verma SK, Singh L (2003). Novel universal primers establish identity of an enormous number of animal species for forensic application. Molecular Ecology Notes3, 28-31. Waits LP, Paetkau D (2005). Noninvasive genetic sampling tools for wildlife biologists: A review of applications and recommendations for accurate data collection. Journal of Wildlife Management69, 1419-33. 66 Texas Tech University, Anthony J. Giordano, May 2015 Wasser SK, Houston CS, Koehler GM, Cadd GG, Fain SR (1997). Techniques for application of faecal DNA methods to field studies of ursids. Molecular Ecology 6, 1091-97. Weber W, Rabinowitz A (1996). A global perspective on large carnivore conservation. Conservation Biology10, 1046-54. Wehausen JD, Ramey II RR, Epps CW (2004). Experiments in DNA extraction and PCR amplification from bighorn sheep feces: the importance of DNA extraction method. Journal of Heredity95, 503-09. White T, Arnheim N, Erlich E (1989). The polymerase chain reaction. Trends in Genetics 5, 185-89. Yahnke CJ, Gamarra de Fox I, Colman F (1998). Mammalian species richness in Paraguay. Biological Conservation84, 263-68. 67 Texas Tech University, Anthony J. Giordano, May 2015 Figure 2.1. Map of Paraguay showing the location of Defenders of the Chaco National Park. 68 Texas Tech University, Anthony J. Giordano, May 2015 69 Texas Tech University, Anthony J. Giordano, May 2015 Table 2.1. Species identification of scat samples by DNA isolation attempt Felid Species ID Cumulative ID Success, n (%) Total (n) Initial Extraction Re-Extraction 1-3 PCR attempts 201 (67.2%) 1-3 PCR attempts 98 (32.8%) 299 Puma (Puma concolor) 129 (67.9%) 61 (32.1%) 190 Small Felids 5 (19.2%)* 21 (80.8%)* 26 Canids 9 (81.8%)* 2 (18.2%)* 11 - - 28 Jaguar (Panthera onca) Indeterminate 554 TOTAL *ATP-6 sequences were lacking for several small cat species in NCBI; due to their non-specificity, all were re-extracted twice to confirm origin 70 Texas Tech University, Anthony J. Giordano, May 2015 Table 2.2. Individual variation in species ID success as determined by laboratory performance in DNA Isolation from scat samples. Performance of Individual Technicians DNA Extractions ID Success Rate (%) Total Number (n) 1 2 3 4 5 6 7 8 87.5 52.7 84.9 50.0 68.0 86.8 48.1 66.7 64 110 126 28 200 76 81 96 71 Texas Tech University, Anthony J. Giordano, May 2015 Table 2.3. Success in sex identification of large felid scat samples by PCR amplification attempt Species Cumulative ID Success Re-Extraction * (n, %) (n, %) 1st PCR 2nd PCR 3rd PCR 4th PCR (2nd + 3rd + 4th) Jaguar Male Female Total 231 (77.3%) 102(44.2) 19(8.3) 15(6.5) 12(5.2) (10+5+5) (8.7) 188(81.4) 19(8.3) 14(6.1) 7(3.0) 4(1.8) (2+2+0)(1.8) 43(18.7)1 Puma Male 39(26.6) 27(11.7) 8(5.4) 5(3.4) (4+3+1)(5.4) 147 (77.4%) 79(53.7) Female 32(21.8) 28(19.1) 6(4.1) 2(1.4) (7+1+0)(5.4) 68(46.3)1 Total 192(50.1) 88(23.3) 36(9.5) 23(6.1) (23+11+6)(10.6) 378 *Whether or not a sample was subject to re-extraction was dependent on the results of attempts to confirm species identification. 1 Amplification attempts presented here do not include additional corroboration for female samples. 72 Texas Tech University, Anthony J. Giordano, May 2015 CHAPTER IV ABUNDANCE & DENSITY OF THE JAGUAR (PANTHERA ONCA) IN THE PARAGUAYAN DRY CHACO: USE OF A NONINVASIVE GENETIC SAMPLING FRAMEWORK ABSTRACT The disappearance of the jaguar (Panthera onca) from many parts of its former range make it an urgent priority for surveys, monitoring, and conservation. Although camera-trapping has been a reliable approach for conducting jaguar surveys, noninvasive genetic sampling (NGS) techniques hold much promise for estimating jaguar abundance and monitoring regional populations. One ecological context suited to this approach is the semi-arid Gran Chaco of Paraguay, where jaguars have received little research or conservation attention to date. We collected 205 jaguar scats across three individual capture-recapture sampling events on roads and trails bordering and transecting Defenders of the Chaco National Park, the country’s largest protected area and a core part of the Gran Chaco Biosphere Reserve. Analysis of 7 microsatellite loci allowed us to identify between 12-19 unique genotypes for each sampling event (PID = 3.65 x 10-3 – 5.67 x 10-3). Using buffers based on the known home ranges of animals from the region, we calculated jaguar density to be D = 0.72/ 100 km2 (95% CI = 0.42 – 0.96/ km2), or one jaguar per 137 km2, in the northwestern Paraguayan Chaco, a relatively low estimate in contrast with findings from the adjacent Bolivian Chaco. This led to an estimate of 58 jaguars (N = 58; 95% CI: 33 – 74) for the Park. We conclude that while NGS is an effective mechanism for monitoring jaguar populations in region so long as it remains relatively cost-effective, differences in jaguar scat sample encounter rates across relatively small heterogeneous regions necessitate the need to sample for jaguars over large areas. We suggest that NGS be conducted quickly for jaguars to minimize potential closure violations, as well as multiple sampling occasions to overcome possible high attrition rates in genotyping success for any one sampling event. Given the importance of the Gran Chaco Jaguar 73 Texas Tech University, Anthony J. Giordano, May 2015 Conservation Unit to rangewide jaguar conservation planning efforts, and the current high rates of deforestation in the Paraguayan Chaco, we recommend improved enforcement of laws protecting jaguars and forest to prevent future population declines, and additional surveys to determine how jaguar density varies across the heterogeneous Gran Chaco-Pantanal mosaic. 74 Texas Tech University, Anthony J. Giordano, May 2015 INTRODUCTION The jaguar (Panthera onca) is the largest obligate carnivore in the western hemisphere. Formerly ranging from the U.S. – Mexico borderlands to northern Patagonia in Argentina, the jaguar now occupies < 46% of its historical distribution (Sanderson et al. 2002). Habitat conversion for cattle ranching and agriculture, the disappearance of prey due to hunting and land development, and direct conflict with landowners over livestock mortality, are the biggest causal factors of declining rangewide jaguar populations (Rabinowitz 1995, 2005; Caso et al. 2008). Jaguars have already been extirpated from Uruguay, El Salvador, and Chile (Caso et al. 2008), no longer occupy nearly 70% of their former range in Mexico and Central America, and have suffered a nearly 90% range contraction in Argentina (Swank & Teer 1989; Caso et al. 2008). Protected areas in the Gran Chaco, approximately one-third of which occurs in Paraguay, potentially represent significant strongholds for jaguar populations. Ranging from southern Bolivia through northcentral Argentina, the Gran Chaco contains the largest contiguous tract of seasonal dry subtropical forest in the western hemisphere (Redford, Taber & Simonetti 1990; Taber, Navarro & Arribas 1997). While recent camera-trapping surveys of the Argentine Chaco failed to record jaguars (Quiroga et al. 2014), earlier field surveys of the Bolivian Chaco suggest jaguar populations may be relatively large and stable there (Maffei, Cuellar & Noss 2004). Together with the Bolivian Chaco, the northern Paraguayan Chaco comprises a large transboundary Jaguar Conservation Unit (JCU), one of the larger JCU’s outside of the Amazon Basin. Jaguar Conservation Units are probable stronghold areas important to the facilitation of rangewide habitat connectivity for jaguars (Sanderson et al. 2002; Rabinowitz & Zeller 2011). However the Paraguayan Chaco, once believed to contain some of the least degraded portions of the Gran Chaco (Redford, Taber & Simonetti 1990), suffers from a complete lack of baseline population data for jaguars. Lack of information on jaguars from this region therefore leaves our picture of the Gran Chaco JCU incomplete. Additionally, past jaguar conservation planning efforts (eg., Sanderson et al. 2002) did not account for recent accelerating deforestation rates in the 75 Texas Tech University, Anthony J. Giordano, May 2015 northern Paraguayan Chaco (Yanosky 2012; Caldas et al. 2013), which may be intensifying conflict between jaguars and people (Giordano 2011). Although most recent investigations of jaguar population parameters have employed camera-trapping techniques (eg., Maffei, Cuellar & Noss 2004; Silver et al. 2004; Silveira et al. 2009; Maffei et al. 2011), wildlife and conservation biologists are increasingly employing the use of noninvasive genetic sampling (NGS). Use of hair traps deployed in fixed spatial arrays emerged as popular early method for genotyping carnivores and ultimately estimating population size (eg., Woods et al. 1999; McDaniel et al. 2000; Frantz et al. 2004). Scats however scats provide an alternative means of identifying individuals (eg., Foran, Crooks & Minta 1997; Ernest et al. 2000; Farrell, Roman & Sunquist 2000; Palomares et al. 2002) and estimating abundance (eg., Kohn et al. 1999; Prugh et al. 2005). Moreover while DNA from hairs of other felid species has been positively identified (eg., Weaver et al. 2005; Castro-Arellano et al. 2008; Kery et al. 2010; Davoli et al. 2013), efforts to sample jaguars in this manner haven’t met with much success (eg., Castro-Arellano et al. 2008). In contrast, genetic differentiation of jaguars from sympatric pumas (Farrell, Roman & Sunquist 2000; Haag et al. 2009) and the genotyping of individual jaguars (Haag et al. 2010; Sollmann et al. 2013) has already proven successful using scats. Scat sampling, though increasingly popular in studying carnivores, is not without distinct technical challenges. Difficulties in overcoming PCR inhibitors for example can present an obstacle to identifying individuals (Creel et al. 2003; Fickel & Hohmann 2006; Beja-Pereira et al. 2009). In tropical environments, DNA amplification success rates of scats can be low (eg., Mukherjee et al. 2010; Michalski et al. 2011), as DNA may degrade quickly due to high humidity and rainfall, direct sunlight, and/or a more decomposers (Waits & Paetkau 2005; Beja-Pereira et al. 2009; Goossens & Salgado-Lynn 2013; Tende et al. 2014). Moreover, low sample encounter rates often necessitate the use of scat detection dogs in an attempt to increase search efficiency and sample sizes (Wasser et al. 2004; Long et al. 2007; MacKay et al. 76 Texas Tech University, Anthony J. Giordano, May 2015 2008), use of which can be both time-intensive and inflate project expenses (Long et al. 2007; MacKay et al. 2008). As the efficacy of techniques to analyze degraded DNA improve and they become more cost-effective, NGS of scats may offer distinct advantages to cameratrapping. Application of scat-based NGS approaches have already proven useful in surveys of carnivores for which individual identification using camera-traps may not be possible (eg., Hansen & Jacobsen 1999; Gompper et al. 2006; Long et al. 2007; Koelewijn et al. 2010; Trinca, Jaeger & Eizirik 2013). The versatility of the NGS approach may also make it better suited to certain species-landscape contexts that present logistical challenges to camera-trapping surveys, making the former more practical. Camera-traps might be more visible in areas where human and vehicle traffic is high, which could lead to increased theft of units (Bancroft 2010; Meek, Ballard & Fleming 2013), or diminished battery life due to frequent triggering. Remote, densely-forested regions, open areas with few trees and natural trails, and generally large geographical regions, might also favor the implementation of a scatbased NGS framework. Given its remoteness, relative lack of infrastructure and trails, and the expansive, dense seasonal deciduous forest that covers much of the region, the Paraguayan Dry Chaco may be particularly suited to NGS of jaguars. Our principle goal here is to provide the first baseline population data for jaguars in the country of Paraguay, and the first estimate of jaguar abundance for the Gran Chaco using a NGS framework. STUDY AREA With a total expanse of > 1,000,000 km2 across Argentina, Paraguay, Bolivia, and a very small portion of south-west Brazil, the Gran Chaco is the second largest contiguous forested biome in the Neotropics outside of the Amazon Basin (Bucher & Huszar 1999). In Paraguay, the Gran Chaco constitutes nearly all of the region west of the Rio Paraguay, or approximately 60% of the country by land area (Figure 1). At approximately 7,800 km2, ‘Defenders of the Chaco’ National Park (DCNP) is Paraguay’s largest protected area (Figure 1), constituting nearly 17% of the Gran 77 Texas Tech University, Anthony J. Giordano, May 2015 Chaco Biosphere Reserve in northern Paraguay. It also accounts for >6% of all xerophytic ‘quebrachal’ or semi-arid scrub and thorn forest in the country (Clark 2012). Seasonal temperatures fluctuate widely throughout the year (0-42°C), and rainfall in the eastern most portion of the park varies between 500-800 mm annually (Campos, Benitez & Merritt, Jr. 2004). METHODS Field Sampling Between April – August 2009, we conducted 3 independent systematic sampling events for large felid scats along approximately 425 km of roads bordering and transecting DCNP in the far northern Dry Chaco of Paraguay (Figure 2). All sampling events were intentionally conducted outside of the Chaco’s core rainy or wet season (December – March) or summer, when flooding can make the area inaccessible. Intervals between sampling events ranged between 2-5 months. All sampling was conducted by traveling road and trail transects using a 4x4 vehicle (< 25 kph) and on foot when necessary. A minimum of 5 pre-trained people,including the driver (i.e., two on each side front and back, with one person in the rear) were stationed in the vehicle when actively searching for samples. Upon locating a scat believed to be from a large felid (i.e., jaguar OR puma), the entire sample was collected, and subsequently annotated and stored in a 1-gallon Ziploc ® sealable plastic bag with silica gel beads following the methods described in Giordano et al. (Chapter 3). Genotyping & Error-Checking PCR primerstargeting the ATP-6 region of the mtDNA genome were used in genetic analyses to distinguish jaguar from puma scats and those of non-target animals (Haag et al. 2009);DNA extraction, PCR amplification, sequencing, and final species identification followed the techniques described by Giordano et al. (Chapter 3). DNA concentration wasn’t quantified but assumed low (cf. Williams et al. 2009). We chose a panel of eight microsatellite loci (FCA77, FCA126, FCA161, FCA211, FCA229, FCA441, FCA453, FCA678), a subset of 29 highly polymorphic loci originally 78 Texas Tech University, Anthony J. Giordano, May 2015 developed for the domestic cat (Menotti-Raymond et al. 1997) but successfully applied to jaguars (Eizirik et al. 2001). PCR amplification (HotStarTaq DNA Polymerase Kit #203203, Qiagen, Inc.) was optimized for a total per sample reaction volume of 10 μl with the following concentration: 1.0 μl of PCR Buffer 10X, 0.4 μl of 25 mM MgCl2, 0.25 μl of 10mM dNTPs, 0.25 μl of 1% BSA (Sigma-Aldrich, Inc.), 0.5 μl of forward primer (1 μM), 0.5 μl of reverse primer (10 μM), 0.5 μl of VIC (green) Dye (10 μM), 0.1 U of HotStarTaq ® at 5 Units /μl, and 3 μl of undiluted template DNA of unknown concentration. Samples were diluted with 3.5 μl distilled water as part of the total final volume and amplified in a PCR Thermocycler machine (Mastercycler ®, Eppendorf, Westbury, NY, USA). The reaction profile consisted of an initial denaturation of 3 minutes at 95°C, followed by 40 cycles (Touchdown) of (1) 95°C of 15 seconds (denaturation), (2) 55°C for 15 seconds (annealing), and (3) 72°C for 30 seconds. A final extension occurred at 72°C for 30 minutes concluded the reaction, and samples were then held at 4°C. All microsatellite loci were subjected to three independent PCR amplification attempts following Huck et al. (2008) and Naidu (2009), and uni-plexed (i.e., amplified individually) with only VIC dye (GelPilot 5x, Qiagen, Inc.). Allele peaks were scored in GENEMARKER ® (SoftGenetics, State College, PA) and corroborated independently by three observers. To mitigate against possible genotyping errors (e.g., allelic dropout, false alleles; cf. Waits & Leberg 2000, Mills et al. 2000; Broquet & Petit 2004; McKelvey & Schwartz 2004) and build consensus genotypes, we only considered samples that were consistent for at least 2 of 3 independent scoring attempts. We calculated the Probability of Identity among full siblings of (PID(sib)) in GENECAP v 1.2 (Wilberg & Dreher 2004) and followed Woods et al. (1999) in setting a threshold PID(sib) of < 0.05 for our population. For each sample, we used GENECAP to compare all alleles at a given locus with alleles at all corresponding loci of all other samples to identify matching genotypes (Wilberg & Dreher 2004). This permitted us to identify potentially problematic genotypes differing by one or two loci allele scores (Wilberg & Dreher 2004). Those genotypes differing by only 1 or 2 loci, where at least one sample was homozygous, as well as 79 Texas Tech University, Anthony J. Giordano, May 2015 some samples where up to two loci could not be genotyped were conservatively considered to be from the same individual provided the new PID(sib) < 0.05 (Woods et al. 1999) for > 6 loci. This conservative approach allowed us to identify ‘ghost’ individuals, or false genotypes (Waits & Leberg 2000; Prugh et al. 2005; Ruell et al. 2009) and avoid overestimates of population size. Samples not meeting these criteria were eliminated from further analyses. Population Size & Density Capture-recapture histories for ‘marked’ (genotyped) samples were organized using output from GENECAP and adapted for each of three independent, 12-14 day single-session sampling events for which we assumed demographic closure. We calculated estimates of jaguar population size and 95% C.I.’s using the “two innate rates of capture” model (TIRM) estimator in capwire, a capture with replacement likelihood estimator that performs better when capture heterogeneity exists (eg., “hard to capture” vs. “easy to capture” individuals), and is robust to small population sizes and low proportional sampling intensity (Miller, Joyce & Waits 2005). To derive density, we used home range area data from 6 jaguars (4 M, 2 F) that were monitored between 2002 and 2005 in the Paraguayan Dry Chaco, including DCNP (A. J. Giordano, unpublished data). We calculated the average radius (AR = 11.425 km) of the 95% kernel of six circle-transformed home ranges in order to buffer sample transects and estimate effective sampling areas for each sampling event. We only buffered transects where the consensus composite genotype of a sample could be resolved and compared to others. In addition, buffer areas overlapping unprotected land bordering the park consisted of little deforestation or modification at the time of sampling, suggesting there was little need to account for large areas containing unsuitable habitat in calculating effective sampling areas. RESULTS We collected a total of 215 jaguar scats across three distinct sampling events (n = 73, 70, 72) in and around DCNP (Table 1). All scats were confirmed as jaguar using 80 Texas Tech University, Anthony J. Giordano, May 2015 the mtDNA ATP-6 gene following the methods and techniques described in Giordano et al. (Chapter 3). Quality Control & Consensus Genotypes We identified locus FCA441 as a potentially problematic microsatellite locus for capture-recapture analysis due to insufficient successful amplification for all three events (23.7%, n=215) eliminated it from further analysis. The Probability of Identity among Siblings (PID-sib) satisfied our minimum threshold requirements (i.e., < 0.05) for 5-7 remaining loci (Table 1), confidently permitting us to distinguish related individuals for population sizes larger than we expected ours to be (cf. Williams et al. 2009). After eliminating all samples where > 2 loci could not be genotyped, we arrived at a multi-locus consensus genotype for 56.3% of all scats (Table 1). Per-locus success rates varied from 62% (FCA 126) to 96% (FCA 161, FCA 210), with a mean success rate of 88% across all loci. We used GENECAP to identify an average of 15.3 unique genotypes (range: 12-19) per sampling event (Table 1). Between 9-14 unique genotypes were ‘captured’ only once among all three sampling events, while the range that the most-captured individuals were ‘captured’ and ‘recaptured’ varied little for all three sampling events (E1max = 5, E1avg = 1.88; E2max = 8, E2avg = 1.95; E3max = 5, E3avg = 1.85). Abundance & Density Estimates of jaguar abundance varied among the three events(N =29-45, 95% CI: 17-42, 26-59) (Table 2). Our highest estimate of jaguar abundance was associated with the event (E2) where we achieved the most success in deriving consensus composite genotypes (68.6%). For each event, the ratio of “difficult-to-capture” individuals (Nb) to “easy-to-capture” individuals (Na) was approximately 3:1, suggesting strong heterogeneity in capture probability. Estimates of jaguar density (D) varied with both estimates of abundance, and the effective sampling area (ESA) used for each sampling event (Table 3). Effective sampling areas varied among sampling events because consensus genotypes could not be identified for samples occurring on some transects. Our most robust estimate of jaguar density (E2) was D = 81 Texas Tech University, Anthony J. Giordano, May 2015 1/137 km2 (95% C.I. = 1/238 km2 – 1/104 km2), or 0.73/ 100 km2 (95% C.I. = 0.42 – 0.96 /100 km2). This suggests the DCNP may host approximately 57 (95% C.I. = 33 – 75) jaguars. DISCUSSION Despite numerous camera-trapping CR studies of felids, Rodgers & Janecka (2013) noted less than 10 scat-based population estimates of felids, though some additional studies targeting pumas and jaguars have occurred since (eg., Sollmann et al. 2013; Miotto et al. 2014; Roques et al. 2014). This study presents the first estimates of jaguar population size for the northwestern Paraguayan Chaco, and the first estimates of jaguar population size for the Gran Chaco that are based on a NGS framework. Identifying Individuals in a NGS Framework One critical assumption underlying CR sampling approaches is that individuals are correctly identified during captures and recaptures, as improper identification can lead to biased estimates (Otis et al. 1978; Amstrup, McDonald & Manly 2005). This makes NGS-based approaches particularly challenging due to potential for PCR failure and genotyping error, the latter of which can originate from ‘shadow’ effects, or the consolidation of different genotypes (cf. Mills et al. 2000) due to too few loci and/or lower allelic diversity, and ‘ghosts’ or false genotypes, which might result from high error rates across a larger number of loci. Both can result in biased estimates, low and high respectively (Taberlet et al. 1996; Taberlet, Waits & Luikart 1999; Mills et al. 2000; Creel et al. 2003; McKelvey & Schwartz 2004; Lukacs & Burnham 2005). Due to high genetic diversity of jaguars in the region (A. J. Giordano, unpublished data), we were more concerned about the potential for ghosts than shadow effects, and so were able to eliminate FCA441 from our original panel of eight loci. In using only four of these 29 loci (PID-sib = 0.02) in bobcats, Ruell et al. (2009) were also more concerned about the potential for ghost genotypes and thus inflated abundance estimates. In sampling fishers (Pekania pennanti) and martens (Martes americana), Williams et al. (2009) acknowledged the trade-offs between ghosts and shadow 82 Texas Tech University, Anthony J. Giordano, May 2015 effects. They initially genotyped sample loci twice and a third time when needed, excluding problematic samples from further analysis using only a subset of six prescreened loci for each species out of a larger original panel. Steinglein et al. (2010) also validated genotypes by conducting multiple independent PCR’s, discarding samples with an adequate PID-sib where < 5 of the 8-9 loci could be successfully scored. Use of Scats With Closed Capture-Recapture Estimators As with camera-trapping and live-trapping approaches, scat sampling events should be short in duration to minimize potential for closure violation and inflating abundance estimates (Otis et al. 1978; White et al. 1982). As our sampling events were completed in less than two weeks, we are confident we satisfied this particular consideration for our target jaguar population. Stenglein et al. (2010) assumed that sampling over month did not lead to closure violations in their collection of scats at gray wolf rendezvous sites. However, the relative age of each sample, ie., time since deposition (see Lukacs & Burnham 2005), is at least one important distinction unique to scat-based NGS CR approaches. Inability to resolve scat age could lead to the incorporation of genotypes from non-resident (i.e., dispersing or transient) individuals, which can violate closure assumptions (Arrendal, Vila & Bjorklund 2007) as if the sampling event had been prolonged. Scats originating from drier conditions may also be more resistant to decay than those deposited in humid habitats, allowing them to persist for longer periods and impacting DNA degradation less (Lindahl 1993; Farrell, Roman & Sunquist 2000; Piggott 2004; Waits & Paetkau 2005; Murphy et al. 2007; Stenglein et al. 2010; Vynne et al. 2011; Goossens & Salgado-Lynn 2013). Similarly, “bleached” (whitened) scats may be more indicative of rapid desiccation and/or mineral content than the age of the scat, or its potential for amplification success (Stenglein et al. 2010; M. Culver, pers. com.). Although clearing an area of scats before beginning can reduce the inclusion of older samples (cf. Ruell et al. 2009; Cariappa 2010), given the size and remoteness of our study area, prior removal of scats would have been logistically infeasible and cost-prohibitive. Moreover, because 83 Texas Tech University, Anthony J. Giordano, May 2015 we suspected that jaguar sign would be relatively low, and our goal was to sample a large geographical region, we collected every scat of potentially large felid origin to maximize our sample size and increase the likelihood of individual recaptures. Janecka et al. (2011) acknowledged the same concerns about inclusion of older scats, but concluded that these samples did not impact their estimates of snow leopard (Panthera uncia) abundance compared with camera-trapping. Jaguar Density in the Gran Chaco Estimates of jaguar density vary considerably across the range of the species (Silver et al. 2004; Astete, Sollmann & Silveira 2008; Maffei et al. 2011; Oliveira et al. 2012). Differences in prey and habitat availability, severity of persecution, and local regulations protecting jaguars are among those factors that can influence local carnivore population density (Gittleman 2001; Karanth & Chellam 2009; Ripple et al. 2014). This can make rangewide or regional conservation planning efforts targeting jaguars challenging. We focused on the DCNP in part because it is the largest protected area in Paraguay, and represents the country’s greatest contiguous expanse of Dry Chaco forest. In addition, accelerating deforestation rates in Paraguay’s Gran Chaco (Giordano 2011; Yanosky 2012; Caldas et al. 2013) and the completion of prior research in Kaa-Iya Gran Chaco National Park and Integrated Management Area (Maffei, Cuellar & Noss 2004) made the need to conduct surveys in the Paraguayan portion of the binational Gran Chaco JCU a priority for assessing the health of this transboundary jaguar population. We believe our most reliable estimates of jaguar density for the northern Paraguayan Chaco are D = 0.73/ 100 km2 (95% C.I.: 0.42 – 0.96/ 100 km2), or 1/ 137 km2 (95% C.I. = 1/238 km2 to 1/104 km2); however while we believe our buffers are realistic, they may be somewhat conservative. Of four jaguars previously radiotracked at the DCNP, two overlapped approximately 20% of their combined total MCP; of the remaining two, one occurred almost completely within the other (A. J. Giordano, unpublished data). Also, differences in sample collection rates among transects across a large geographical region, and the potential for individual animals to 84 Texas Tech University, Anthony J. Giordano, May 2015 vary nonrandomly in DNA quality and quantity (Lukacs & Burnham 2005) could compound bias associated with capture heterogeneity, particularly if “difficult-tocapture” individuals on transects with little sample representation were consistently genotyped less frequently. Our data appear to suggest one of these factors may be at work among our samples. For example, although the number of jaguar samples we collected varied little among sampling events (70-73), there appeared to be a considerable range in the proportion of samples for which consensus genotypes could be identified (43.8 - 68.6%), perhaps due to variation in field conditions (Giordano et al., Chapter 3). As a result, some jaguar samples from less productive transects could not be genotyped. Unsurprisingly, the sampling event yielding the greatest number of consensus genotypes (E2) produced the highest estimates of abundance, suggesting that genotyping success rates from other events may have underrepresented the number of unique individuals among samples collected. Overall, approximately 43% of our samples identified as jaguar did not yield a multi-locus consensus genotype. Our estimates of jaguar density for the northwestern Paraguayan Chaco is in contrast with estimates from the Bolivian Chaco. Maffei, Cuellar & Noss (2004) concluded jaguar densities there were several times higher than ours (1/ 45 km2) for Kaa-Iya, a protected area in Bolivia more than four times the size of DCNP. Because parts of Kaa-Iya are geographically very close (<60 km) to DCNP, both parks are part of the same greater Dry Chaco landscape. That regional estimates differ however is not surprising, and might be due to a number of factors. For example, our data were collected approximately 6-8 years later than Maffei, Cuellar & Noss (2004), and in a different socio-political context with land use practices and rates of land use change unique to Paraguay. Deforestation and land conversion, and intensifying humanjaguar conflict is likely high in parts of the Paraguayan Chaco (Giordano 2011; Yanosky 2012; Caldas et al. 2013), possibly causing jaguars to avoid human activity, and/or resulting in a third ‘class’ of unsampled jaguars. For example, although the number of ‘difficult-to-capture’ vs. ‘easy-to-capture’ individual jaguars were consistently estimated in an approximately 3:1 ratio at DCNP, it is possible that some jaguars don’t or hardly use roads, which would bias our estimates low. As our 85 Texas Tech University, Anthony J. Giordano, May 2015 sampling coincided with periods of increased seasonal traffic relating to commercial cattle transport, this may have been a factor. Ruell et al. (2009) found substantial evidence of capture heterogeneity in a bobcat population surrounded by human development in southern California. More generally, human presence and activity is known to negatively impact the activity, habitat use, and distribution of many large carnivores (Ripple et al. 2014). Compared to DCNP, Kaa-Iya might better insulate jaguars from the impacts of human presence and activity. Also,female jaguars avoided roads more than males in the Mexican Yucatan (Conde et al. 2010) and across the jaguar’s range, females appear to be underrepresented in scats (Palomares et al. 2012). In our study area, females also represent a ‘class’ of jaguars sampled proportionally much less than males (Giordano et al., Chapter 3), and this could have biased our density estimates low. Spatiotemporal variation in the distribution of prey use could impact scat deposition rates among transects (Giordano 2005), and social structure might also impact heterogeneity in use or deposition of scats on roads, or their visibility to researchers (i.e., location). Non-resident, transient felids for example may be less likely to deposit scats on roads, or make a greater effort to hide their presence, in the territories of residents; conversely, resident male felids mark conspicuously to signal territorial boundaries to conspecifics (Kitchener 1991; Laing & Lindzey 1993; Sunquist & Sunquist 2002). Different sampling methods can also result in contrasting estimates of jaguar density. In the semi-arid caatinga, Sollmann et al. (2013) used a spatial CR approach to calculate a slightly higher jaguar density estimate for a scat-based approach (2.03/100 km2) compared to camera-trapping (1.45/100 km2), and approaches incorporating both sampling techniques may be a means to improve large felid abundance estimates (Gopalaswamy et al. 2012; Sollmann et al. 2013). However, social structure could also result in scat-based approaches yielding lower estimates compared to camera-trapping. Transient jaguars for example might leave behind fewer scats in resident territories, though still be recorded by camera-traps. Use of different estimators, such as the jackknife estimator (MH) and an SCR approach, could also exacerbate differences in estimates, as well as their precision, for the same dataset 86 Texas Tech University, Anthony J. Giordano, May 2015 (eg., Maffei, Cuellar & Noss 2004; Noss et al. 2012). Williams et al. (2009) suggested that use of multiple traditional CR sessions to analyze NGS data in Program MARK may have underestimated population size for two mesocarnivores relative to capwire estimators. Our choice of capwire’s TIRM (MTIR) was based on the expected low functional sample sizes due to attrition in genotyping success, the need to collapse CR data into a single session, and the proportionally low sampling intensity we anticipated. MTIR appears to exhibit relatively greater precision and less negative bias than other estimators when capture heterogeneity exists, as well as at smaller population sizes (i.e., < 100) and lower sampling intensities (Miller, Joyce & Waits 2005; Puechmaille & Petit 2007). We achieved a sampling intensity of approximately 1.85 – 1.95 samples/ individual, well above the 1.6 samples/ individual below which Stenglein et al. (2010) observed a decline in estimator performance. Overestimates of population size can result if the average home range size of individuals sampled are proportionately large relative to the ESA (Otis et al. 1978; White et al. 1982; Amstrup, McDonald & Manly 2005). In estimating jaguar density in the caatinga of Brazil, Silveria et al. (2009) sampled approximately 50% of the 1291 km2 Serra da Capivara National Park and were confident in applying their jaguar density estimates to the entire protected area. By incorporating known home range data, we were able to approximate a relatively large ESA for our study. Our contiguous ESA for D = 1/137 km2 was almost 80% of the size of DCNP (i.e., 6173 km2). This was approximately six times larger than the cumulative ESA of five discontinuous camera-trapping grids employed by Maffei, Cuellar & Noss (2004) in Kaa-Iya, a protected area more than four times larger than DCNP. Jaguar density estimates for the entire park then were based on sampling only 3% of Kaa-Iya’s area, which could be of consequence to conservation planning efforts. In addition, the KaaIya landscape differs from the DCNP in that hydrocarbon exploration, extraction and transport (Winer 2003), as well as cattle-grazing (Taber et al. 1997), are permitted within its borders, potential considerations for sampling jaguars. Heterogeneity in land use practices and habitat must be a consideration when applying density estimates from smaller sampling areas more broadly, reinforcing the need to sample over 87 Texas Tech University, Anthony J. Giordano, May 2015 proportionally larger areas for jaguars, as impractical as this may be. Alternatively, a re-examination of data from Kaa-Iya with more robust methods like SCR (Noss et al. 2012) yielded a wider range of density estimates (0.31 – 1.82/ 100 km2, or 1/322 km2 to 1/55 km2), likely more suggestive of the variability in jaguar density across the Gran Chaco, and overlapping ours for DCNP (0.42 – 0.96/ 100 km2). Even when considering our highest estimates in the 95% CI (ie., D = 0.96 jaguars/ 100 km2 , or 1/105 km2), ours are among the lowest densities recorded for jaguars across their range. They are comparable to density estimates from the Upper Parana Atlantic Forest fragments of Argentina (Paviolo et al. 2008; D = 0.49 – 1.07/ km2). However, our estimates are consistent with known home range (95% kernel) data from jaguars in the region, and we are reasonably confident in their validity. Relatively low jaguar densities may reflect low densities of medium-large prey, a factor strongly deterministic of tiger abundance (Karanth et al. 2004). Low density of prey in the DCNP may be due to the park’s seasonal aridity and low mean annual precipitation (Gorham 1973; Campos, Benitez & Merritt, Jr. 2004), which may lead to comparatively less plant productivity (Davidson 1977). Silveira et al. (2009) suggested this was factor influencing jaguar density in the Brazilian caatinga, an ecoregion floristically similar to the Dry Chaco but with a higher jaguar density. Finally, we advise caution in the application of our jaguar density estimates to other portions of the Paraguayan Chaco, even areas in relative geographical proximity to the DCNP. This is due in part to the very heterogeneous nature of Gran Chaco forests and grasslands (Navarro, Molina, & de Molas 2006; Spichiger & Ramella 1989). For example, while large portions of the partially contiguous and similarly large Chaco Médanos National Park may contain forest habitat similar to DCNP, habitat suitability for jaguars may vary considerably across the park due to expansive areas of high aridity. In addition, the home ranges (95% kernel) of two jaguars at and near Rio Negro National Park, a region in the Paraguayan Pantanal approximately 160 km away from DCNP, were only 20% of the home ranges of jaguars from the Dry Chaco (A. J. Giordano, unpublished data). This may be indicative of large changes to 88 Texas Tech University, Anthony J. Giordano, May 2015 jaguar density possibly occurring over a relatively short geographical distance. Jaguar densities in the Paraguayan Pantanal could approach that of the contiguous Brazilian Pantanal, which are among the highest on record (Soisalo &Cavalcanti 2006). Given the critical status of jaguars in the Argentine Chaco (Quiroga et al. 2014), and the possible erosion of their range in the Chaco along the Paraguay-Argentina border (Giordano et al. 2014), the role of the Gran Chaco and Pantanal JCU’s to the jaguar’s overall conservation statusmust take on new importance. That vast areas of the Paraguayan Chaco forest still might not protect viable jaguar populations in the longterm should reinforce the urgent need to establish additional protected areas across the region, as well as underscore the importance of slowing the rapid spread of deforestation within the Gran Chaco Biosphere Reserve (Yanosky 2012; Caldas et al. 2013). 89 Texas Tech University, Anthony J. Giordano, May 2015 REFERENCES Amstrup, S.C., McDonald, T.L. & Manly, B.F.J. (2005) Handbook of capturerecapture analysis. Princeton University Press, Princeton, New Jersey, USA. Arrendal, J., Vila, C., & Bjorklund, M. (2007) Reliability of noninvasive genetic census of otters compared to field censuses. Conservation Genetics, 8, 10971107. Astete, S., Sollmann, R. & Silveira, L. (2008) Comparative ecology of jaguars in Brazil. Cat News, Special Issue, 4, 9-14. Bancroft, P. (2010) Property surveillance and security. Deer cameras: the science of scouting (ed L. Thomas), pp. 222-231. Quality Deer Management Association, Bogart, GA, USA. Beja-Pereira, A., Oliveira, R., Alves, P., Schwartz, M.K. & Luikart, G. (2009) Advancing ecological understandings through technological transformations in noninvasive genetics. Molecular Ecology Resources, 9, 1279-1301. Broquet, T. & Petit, E. (2004) Quantifying genotyping errors in noninvasive population genetics. Molecular Ecology, 13, 3601-3608. Bucher, E.H. & Huszar, P.C. (1999) Sustainable management of the Gran Chaco of South America: ecological promise and economic constraints. Journal of Environmental Management, 57, 99-108. Caldas, M.M., Goodin, D., Sherwood, S., Campos Krauer, J.M. & Wisely, S.M. (2013) Land-cover change in the Paraguayan Chaco: 2000-2011. Journal of Land Use Science,DOI: 10.1080/1747423X.2013.807314. Campos, J.M., Benitez, I. & Merritt, Jr, D.A. (2004) On the occurrence of the owl monkey (Aotus azarai) in Cerro Leon, Chaco, Paraguay. Neotropical Primates, 12, 55-56. Cariappa, C. (2010) Three excursions into the ecology of wolves. PhD Dissertation, Texas Tech University, Lubbock, TX. Caso, A., Lopez-Gonzalez, C., Payan, E., Eizirik, E., Oliveira, T.G., Leite-Pitman, R., Kelly, M. & Valderrama, C. (2008) Panthera onca. IUCN 2013. IUCN Red 90 Texas Tech University, Anthony J. Giordano, May 2015 List of Threatened Species. Version 2013.2. Electronic database accessible at http://www.iucnredlist.org/. Accessed on 15 October 2013. Castro-Arellano, I., Madrid-Luna, C., Lacher, Jr., T.E. & Leon-Paniagua, L. (2008) Hair-trap efficacy for detecting mammalian carnivores in the tropics. Journal of Wildlife Management, 72, 1405-1412. Clark, P.T. (2012) Guide to Paraguay’s National Parks and Other Protected Areas, Revised 2nd edition. Printed and Published by Peter T. Clark, Asuncion, Paraguay. Conde, D.A., Colchero, F., Zarza, H., Christensen, Jr., N.L., Sexton, J.O., Manterola, C., Chavez, C., Rivera, A., Azuara, D. & Ceballos, G. (2010) Sex matters: modeling male and female habitat differences for jaguars conservation. Biological Conservation, 143, 1980-1988. Creel, S., Spong, G., Sands, J.L., Rotella, J., Zeigle, J., Joe, L., Murphy, K.M. & Smith, D. (2003) Population size estimation in Yellowstone wolves with errorprone noninvasive microsatellite genotypes. Molecular Ecology, 12, 20032009. Davidson, D.W. (1977) Species diversity and community organization in desert seedeating ants. Ecology, 58, 711-724. Davoli, F., Schmidt, K., Kowalczyk, R. & Randi, E. (2013) Hair snaring and molecular genetic identification for reconstructing the spatial structure of Eurasian lynx populations. Mammalian Biology, 78, 118-126. Eizirik, E., Kim, J.-H., Menotti-Raymond, M., Crawshaw, P.G., O’Brien, S.J. & Johnson, W.E. (2001) Phylogeography, population history, and conservation genetics of jaguars (Panthera onca, Mammalia, Felidae). Molecular Ecology, 10, 65-79. Ernest, H.B., Penedo, M.C.T., May, B.P., Syvanen, M. & Boyce, W.M. (2000) Molecular tracking of mountain lions in the Yosemite Valley region in California: genetic analysis using microsatellites and faecal DNA. Molecular Ecology, 9, 433-441. 91 Texas Tech University, Anthony J. Giordano, May 2015 Farrell, L.E., Roman, J. & Sunquist, M.E. (2000) Dietary separation of sympatric carnivores identified by molecular analysis of scats. Molecular Ecology, 9, 1583-1590. Fickel, J., & Hohmann, U. (2006) A methodological approach for non-invasive sampling for population size estimates in wild boar (Sus scrofa). European Journal of Wildlife Research, 52, 28-33. Foran, D.F., Crooks, K.R. & Minta, S.C. (1997) Species identification from scat: an unambiguous genetic method. Wildlife Society Bulletin, 25, 835-839. Frantz, A.C., Schaul, M., Pope, L.C., Fack, F., Schley, L., Muller, C.P. & Roper, T.J. (2004) Estimating population by genotyping remotely plucked hair: the Eurasian badger. Journal of Applied Ecology, 41, 985-995. Giordano, A.J. (2005) Feeding ecology of a reintroduced river otter (Lontra canadensis) population in northcentral Pennsylvania. MSc Thesis, Frostburg State University, Frostburg, MD. Giordano A.J. (2011) Estado y conservación del jaguar (Panthera onca) en el Chaco Paraguayo. El futuro del jaguar en el Gran Chaco – Situacion en Bolivia, Paraguay, and Argentina, eds D. I. Rumiz, J. Polisar, & L. Maffei, pp. 17-18. Wildlife Conservation Society, Santa Cruz, Bolivia. Giordano, A.J., Mujica Cameroni, N., Ramirez, F., and Nielsen, C.K. (2014) Jaguar records from the south-central Paraguayan Chaco. Cat News, 60, 38-40 Giordano, A.J., Vargas, K.L., Wisely, E., Gipson, P., Wallace, M. & Culver, M. In prep [Chapter 3]. Adaptation of noninvasive genetic techniques for identifying jaguars (Panthera onca) and pumas (Puma concolor) in the Gran Chaco: practical considerations and implications for sampling. Gittleman, J.L., Funk, S., Macdonald, D.W. & Wayne, R.K. (2001) Carnivore Conservation. Cambridge University Press, Cambridge. Gompper, M.E., Kays, R.W., Ray, J.C., LaPoint, S.D., Bogan, D.A. & Cryan, J.R. (2006) A comparison of noninvasive techniques to survey carnivore communities in northeastern North America. Wildlife Society Bulletin, 34, 1142-1151. 92 Texas Tech University, Anthony J. Giordano, May 2015 Goossens, B. & Salgado-Lynn, M. (2013) Advances and difficulties of molecular tools for carnivore conservation in the tropics. Raffles Bulletin of Zoology, Supplement 28, 43-53. Gopalaswamy, A.M., Royle, J.A., Delampady, M., Nichols, J.D., Karanth, K.U. & Macdonald, D.W. (2012) Density estimation in tiger populations: combining information for strong inference. Ecology, 93, 1741-1751. Gorham, J.R. (1973) The Paraguayan Chaco and its rainfall. Paraguay Ecological Essays, ed J.R. Gorham, pp. 39-60. Academy of the Arts and Sciences of the Americas, Miami, FL. Haag, T., Santos, A.S., De Angelo, C., Srbek-Araujo, A.C., Sana, D.A., Morato, R.G., Salzana, F.M. & Eizirik, E. (2009) Development and testing of an optimized method for DNA-based identification of jaguar (Panthera onca) and puma (Puma concolor) faecal samples for use in ecological and genetic studies. Genetica, 136, 505-512. Haag, T., Santos, A.S., Sana, D.A., Morato, R.G., Cullen, Jr., L., Crawshaw, Jr. P.G., De Angelo, C., Di Bitetti, M.S., Salzano, F.M. & Eizirik, E. (2010) The effect of habitat fragmentation on the genetic structure of a top predator: loss of diversity and high differentiation among remnant populations of Atlantic Forest jaguars. Molecular Ecology, 19, 4906-4921. Hansen, M. M. & Jacobsen, L. (1999) Identification of mustelid species: otter (Lutra lutra), American mink (Mustela vison) and polecat (Mustela putorius), by analysis of DNA from faecal samples. Journal of Zoology, 247, 177-181. Huck, M., Frantz, A.C., Dawson, D.A., Burke, T. & Roper, T.J. (2008) Low genetic variability, female-biased dispersal and high movement rates in an urban population of Eurasian badgers Meles meles. Journal of Animal Ecology, 77, 905-915. Janecka, J.E., Munkhtsog, B., Jackson, R.M., Naranbaatar, G., Mallon, D.P. & Murphy, W.J. (2011) Comparison of noninvasive genetic and camera-trapping techniques for surveying snow leopards. Journal of Mammalogy, 92, 771-783. 93 Texas Tech University, Anthony J. Giordano, May 2015 Karanth, K.U., Nichols, J.D., Kumar, N.S., Link, W.A. & Hines, J.E. (2004) Tigers and their prey: predicting carnivore densities from prey abundance. Proceedings of the National Academy of Science, 101, 4854-4858. Karanth, K. U. & Chellam, R. (2009) Carnivore conservation at the crossroads. Oryx, 43, 1-2. Kery, M., Gardner, B., Stoeckle, T., Weber, D. & Royle, J.A. (2010) Use of spatial capture-recapture modeling and DNA data to estimate densities of elusive animals. Conservation Biology, 25, 356-364. Kitchener, A. (1991) The Natural History of the Wild Cats. Christopher Helm, London, UK. Koelewijn, H.P., Perez-Haro, M., Jansman, H.A.H., Boerwinkel, M.C., Bovenschen, J., Lammertsma, D.R., Niewold, F.J.J. & Kuiters, A.T. (2010) The reintroduction of the Eurasian otter (Lutra lutra) into the Netherlands: hidden life revealed by noninvasive genetic monitoring. Conservation Genetics, 11, 601-614. Kohn, M.H., York, E.C., Kamradt, D.A., Haught, G., Sauvajot, R.M. & Wayne, R.K. (1999) Estimating population size by genotyping faeces. Proceedings of the Royal Society of London B, 266, 657-663. Laing, S.P. & Lindzey, F.G. (1993) Patterns of replacement of resident cougars in southern Utah. Journal of Mammalogy, 74, 1056-1058. Lindahl, T. (1993) Instability and decay of the primary structure of DNA. Nature, 362, 709-715. Long, R.A., Donovan, T.M., MacKay, P., Zielinski, W.J. & Buzas, J.B. (2007) Comparing scat detection dogs, cameras, and hair snares for surveying carnivores. Journal of Wildlife Management, 71, 2018-2025. Lukacs, P.M. & Burnham, K.P. (2005) Review of capture-recapture methods applicable to noninvasive genetic sampling. Molecular Ecology, 14, 39093919. 94 Texas Tech University, Anthony J. Giordano, May 2015 MacKay, P., Smith, D.A., Long, R.A. & Parker, M. (2008) Noninvasive survey methods for carnivores (eds R.A. Long, P. MacKay, W.J. Zielinski & J.C. Ray), pp. 183-221. Island Press, Washington, DC. Maffei, L., Cuellar, E., & Noss, A. (2004) One thousand jaguars (Panthera onca) in Bolivia’s Chaco? Camera trapping in the Kaa-Iya National Park. Journal of Zoology, 262, 295-304. Maffei, L., Noss, A.J., Silver, S.C. & Kelly, M.J. (2011) Abundance/ density case study: jaguars in the Americas. Camera Traps in Animal Ecology, eds A.F. O’Connell, J.D. Nichols & K.U. Karanth, pp. 119-144.. Springer Japan, Tokyo, Japan. McDaniel, G. W., McKelvey, K.S., Squires, J.R. & Ruggiero, L.F. (2000) Efficacy of lures and hair snares to detect lynx. Wildlife Society Bulletin, 28, 119-123. McKelvey, K.S. & Schwartz, M.K. (2004) Genetic errors associated with population estimation using non-invasive molecular tagging: problems and new solutions. Journal of Wildlife Management, 68, 439-448. Meek, P.D., Ballard, G.-A. & Fleming, P.J.S. (2013) A permanent security post for camera trapping. Australian Mammalogy, 35, 123-127. Menotti-Raymond, M., David, V.A., Stephens, J.C., Lyons, L.A. & S.J. O’Brien. (1997) Genetic individualization of domestic cats using feline STR loci for forensic application. Journal of Forensic Science, 42, 1039-1051. Michalski, F., Valdez F.P., Norris, D., Ziemiski, C., Kashivakura, C.K., Trinca, C.S., Smith, H.B., Vynne, C., Wasser, S.K., Metzger, J.P. & Eizirik, E. (2011) Successful carnivore identification with faecal DNA across a fragmented Amazonian landscape. Molecular Ecology Resources, 11, 862-871. Miller, C.R., Joyce, P. & Waits, L.P. (2005) A new method for estimating the size of small populations from genetic mark-recapture data. Molecular Ecology, 14, 1991-2005. Mills, L.S., Citta, J.J., Lair, K.P., Schwartz, M.K. & Tallmon, D.A. (2000) Estimating animal abundance using noninvasive DNA sampling: promise and pitfalls. Ecological Applications, 10, 282-294. 95 Texas Tech University, Anthony J. Giordano, May 2015 Miotto, R.A., Cervini, M., Kajin, M., Begotti, R.A. & Galetti, Jr, P.M. (2014) Estimating puma Puma concolor population size in a human-dominated landscape in Brazil, using DNA mark-recapture. Oryx, 48, 250-257. Mukherjee, S., Ashalakshmi, C.N., Home, C. & Ramakrishnan, U. (2010) An evaluation of the PCR-RFLP technique to aid molecular-based monitoring of felids and canids in India. BMC Research Notes, 3, 159. http//www.biomedcentral.com/1756-0500/3/159. Murphy, M.A., Kendall, K.C., Robinson, A. & Waits, L.P. (2007) The impact of time and field conditions of brown bear (Ursus arctos) fecal DNA amplification. Conservation Genetics, 8, 1219-1224. Naidu, A. (2009) Genetic analysis of mountain lion (Puma concolor) feces from Kofa National Wildlife Refuge, Arizona. MSc Thesis, University of Arizona, Tucson, AZ. Navarro, G., Molina, J.A. & de Molas, L.P. (2006) Classification of the forests of the northern Paraguayan Chaco. Phytocoenologia, 36, 473-508. Noss, A.J., Gardner, B., Maffei, L., Cuellar, E., Montano, R., Romero-Munoz, A., Sollman, R. & O’Connell, A.F. (2012) Comparison of density estimation methods for mammal populations with camera traps in the Kaa-Iya del Gran Chaco landscape. Animal Conservation, 15, 527-535. Oliveira, T.G. de, Ramalho, E.E. & de Paula, R.C. (2012) Red list assessment of the jaguar in Brazilian Amazonia. Cat News, Special Issue, 7, 8-13. Otis, D.L., Burnham, K.P., White, G.C. & Anderson, D.R. (1978) Statistical inference from capture data on closed animal populations. Wildlife Monographs 62, The Wildlife Society, Bethesda, MD. Palomares, F., Godoy, J.A., Piriz, A., O’Brien, S.J. & Johnson, W.E. (2002) Faecal genetic analysis to determine the presence and distribution of elusive carnivores: design and feasibility for the Iberian lynx. Molecular Ecology, 11, 2171-2182. Palomares, F., Roques, S., Chavez, C., Silveira, L., Keller, C., Sollmann, R., do Prado, D.M., Torres, P.C., Adrados, B., Godoy, J.A., de A. Jacomo, A.T., Torres, 96 Texas Tech University, Anthony J. Giordano, May 2015 N.M., Furtado, M.M. & Lopez-Bao, J.V. (2012) High proportion of male feces in jaguar populations. PLOS One, 7(12), e52923. Paviolo, A., De Angelo, C.D., Di Blanco, Y.E. & Di Bitetti, M.S. (2008) Jaguar Panthera onca population decline in the Upper Parana Atlantic Forest of Argentina and Brazil. Oryx, 42, 554-561. Piggott, M.P. (2004) Effect of sample age and season of collection on the reliability of microsatellite genotyping of faecal DNA. Wildlife Research, 31, 485-493. Prugh, L.R., Ritland, C.E., Arthur, S.M., Krebs, C.J. (2005) Monitoring coyote population dynamics by genotyping faeces. Molecular Ecology, 14, 15851596. Puechmaille, S.J, & Petit, E.J. (2007) Empirical evaluation of non-invasive capturemark-recapture estimation of population size based on a single sampling session. Journal of Applied Ecology, 44, 843-852. Quiroga, V.A., Boaglio, G.I., Noss, A.J. & Di Bitetti, M.S. (2014) Critical population status of the jaguar (Panthera onca) in the Argentina Chaco: camera-trap surveys suggest recent collapse and imminent regional extinction. Oryx, 48, 141-148. Rabinowitz, A. (1995) Jaguar conflict and conservation: a strategy for the future. Integrating People and Wildlife for a Sustainable Future (eds J.A. Bissonette & P.R. Krausman), pp. 394-397. The Wildlife Society, Bethesda, MD. Rabinowitz, A. (2005) Jaguars and livestock: living with the world’s largest cat. People and Wildlife: Conflict or Coexistence? (eds R. Woodroffe, S. Thirgood & A. Rabinowitz), pp. 278-285. Cambridge University Press, Cambridge. Rabinowitz, A. & Zeller, K. (2011) A range-wide model of landscape connectivity for the jaguar, Panthera onca. Biological Conservation, 143, 939-945. Redford, K.H., Taber, A. & Simonetti, J.A. (1990) There is more to biodiversity than the tropical rain forests. Conservation Biology, 4, 328-330. Ripple, W.J., Estes, J.A., Beschta, R.L., Wilmers, C.C., Ritchie, E.G., Hebblewhite, M., Berger, J., Elmhagen, B., Letnic, M., Nelson, M.P., Schmitz, O.J., Smith, 97 Texas Tech University, Anthony J. Giordano, May 2015 D.W., Wallach, A.D., & Wirsing, A.J. (2014) Status and ecological effects of the world’s largest carnivores. Science, 343, DOI: 10.1126/science.1241484. Rodgers, T.W. & Janecka, J.E. (2013) Applications and techniques for non-invasive genetics research in felid conservation. European Journal for Wildlife Research, 59, 1-16. Roques, S., Furtado, M., Jacomo, A.T.A., Silveira, L., Sollmann, R., Torres, N.M., Godoy, J.A. & Palomares, F. (2014) Monitoring jaguar populations Panthera onca with non-invasive genetics: a pilot study in Brazilian ecosystems. Oryx, DOI: 10.1017/S0030605312001640. Ruell, E.W., Riley, S.P.D., Douglas, M.R., Pollinger, J.P. & Crooks, K.R. (2009) Estimating bobcat population sizes and densities in a fragmented landscape using noninvasive capture-recapture sampling. Journal of Mammalogy, 90, 129-135. Sanderson, E.W., Redford, K.H., Chetkiewicz, C-L.B., Medellin, R.A., Rabinowitz, A.R., Robinson, J.G. & Taber, A.B. (2002) Planning to save a species: the jaguar as a model. Conservation Biology, 16, 58-72. Silver, S., Ostro, L.E.T., Marsh, L.K., Maffei, L., Noss, A.J. & Kelly, M. (2004) The use of camera traps for estimating jaguar (Panthera onca) abundance and density using capture/recapture analysis. Oryx, 38, 148-154. Silveira, L., A.T.A. Jacomo, Astete, S., Sollmann, R., Torres, N.M., Furtado, M.M. & Marinho-Filho, J. (2009) Density of the Near Threatened jaguar Panthera onca in the caatinga of north-eastern Brazil. Oryx, 44, 104-109. Soisalo, M. & Cavalcanti, S. (2006) Estimating the density of a jaguar population the Brazilian Pantanal using camera-traps and capture-recapture sampling in combination with GPS radio-telemetry. Biological Conservation, 129, 487496. Sollmann, R., Torres, N.M., Furtado, M.M., Jacomo, A.T.A., Palomares, F., Roques, S. & Silveira, L. (2013) Combining camera-trapping and noninvasive genetic data in spatial capture-capture framework improves density estimates for the jaguar. Biological Conservation, 167, 242-247. 98 Texas Tech University, Anthony J. Giordano, May 2015 Spichiger, R., & Ramella, L. (1989) The forests of the Paraguayan Chaco. Tropical Forests: Botanical Dynamics, Speciation, and Diversity (eds L.B. HolmNielsen, I. C. Nielsen & H. Balslev), pp. 259-270. Academic Press, London. Stenglein, J.L., Waits, L.P., Ausband, D.E., Zager, P. & Mack, C.M. (2010) Efficient noninvasive genetic sampling for monitoring reintroduced wolves. Journal of Wildlife Management, 74, 1050-1058. Sunquist, M. & Sunquist, F. (2002) Appendix 3. Olfactory communication in felids. Wild Cats of the World (eds M. Sunquist & F. Sunquist), pp. 413-420. University of Chicago Press, Chicago, IL. Swank, R.K. & Teer, J.G. (1989) Status of the jaguar – 1987. Oryx, 23, 14-21. Taber, A.B., Navarro, G., & Arribas, M.A. (1997) A new park in the Bolivian Gran Chaco – an advance in tropical dry forest conservation and community-based management. Oryx, 31, 189-198. Taberlet, P., Griffin, S., Goossens, B., Questiau, S., Manceau, V., Escaravage, N., Waits, L.P. & Bouvet, J. (1996) Reliable genotyping of samples with very low DNA quantities using PCR. Nucleic Acids Research, 24, 3189-3194. Taberlet, P., Waits, L.P. & Luikart, G. (1999) Noninvasive genetic sampling: look before you leap. Trends in Ecology and Evolution, 14, 323-327. Tende, T., Hansson, B., Ottosson, U., & Bensch, S. (2014) Evaluating preservation medium for the storage of DNA in African lion Pantheraleo faecal samples. Current Zoology, 60, 351-358. Trinca, C.S., Jaeger, C.F. & Eizirik, E. (2013) Molecular ecology of the Neotropical otter (Lontra longicaudis): non-invasive sampling yields insights into local population dynamics. Biological Journal of the Linnean Society, 109, 932-948. Vynne, C., Baker, M.R., Breuer, Z.K. & Wasser, S.K. (2011) Factors influencing degradation of DNA and hormones in maned wolf scat. Animal Conservation, 15, 184-194. Waits, L.P. & Leberg, P.L. (2000) Biases associated with population estimation using molecular tagging. Animal Conservation, 3, 191-200. 99 Texas Tech University, Anthony J. Giordano, May 2015 Waits, L.P. & Paetkau, D. (2005) Noninvasive genetic sampling tools for wildlife biologists: A review of applications and recommendations for accurate data collection. Journal of Wildlife Management, 69, 1419-1433. Wasser, S.K., Davenport, B., Ramage, E.R., Hunt, K.E., Parker, M., Clarke, C. & Stenhouse, G. (2004) Scat detection dogs in wildlife research and management: application to grizzly and black bears in the Yellowhead Ecosystem, Alberta, Canada. Canadian Journal of Zoology, 82, 475-492. Weaver, J.L., Wood, P., Paetkau, D. & Laack, L.L. (2005) Use of scented hair snares to detect ocelots. Wildlife Society Bulletin, 33, 1384-139. White, G.C., Anderson, D.R., Burnham, K.P., & Otis, D.L. (1982) Capture-recapture and removal methods for sampling closed populations. LA-8787-NERP:1-235. Los Alamos National Laboratory, Los Alamos, NM. Wilberg, M.L. & Dreher, B.D. (2004) GENECAP: a program for analysis of multilocus genotype data non-invasive sampling and capture-recapture population estimation. Molecular Ecology Notes, 4, 783-785. Williams, B.W., Etter, D.R., Linden, D.W., Millenbah, K.F., Winterstein, S.R. & Scribner, K.T. (2009) Noninvasive hair sampling and genetic tagging of codistributed fishers and American martens. Journal of Wildlife Management, 73, 26-34. Winer, N. (2003) Co-management of protected areas, the oil and gas industry and indigenous empowerment – the experience of Bolivia’s Kaa-Iya del Gran Chaco. Policy Matters (IUCN CEESP), 12, 181-191. Woods, J.G., Paetkau, D., Lewis, D., McLellan, B.N., Proctor, M. & Strobeck, C. (1999) Genetic tagging of free-ranging black and brown bears. Wildlife Society Bulletin, 27, 616-627. Yanosky, A. (2012) The challenge of conserving a natural Chaco habitat in the face of severe deforestation pressure and human development needs. The Paraguay Reader: History, Culture, Politics (eds P. Lambert & A. Nickson), pp. 376382. Duke University Press, Durham, NC. 100 Texas Tech University, Anthony J. Giordano, May 2015 Figure 3.1. Coarse boundary map for the general ecoregions of Paraguay, showing the location of Defenders of the Chaco National Park (black) in the northern dry Chaco. 101 Texas Tech University, Anthony J. Giordano, May 2015 102 Texas Tech University, Anthony J. Giordano, May 2015 Figure 3.2. The portions of roads bordering and transecting DCNP serving as regular jaguar scat sampling transects for all systematic sampling events. 103 Texas Tech University, Anthony J. Giordano, May 2015 104 Texas Tech University, Anthony J. Giordano, May 2015 Table 3.1. Jaguar scat samples collected (April – August 2009), % of samples successfully yielding multi-locus consensus genotypes, unique genotypes, and the Probability of Identity for Siblings for each CR-NGS event at DCNP. Event Samples Collected (n) CG (%) Unique Genotypes1 P(ID)-sib 1 73 43.8% 12 3.65 x 10-3 2 70 68.6% 19 5.67 x 10-3 3 72 56.9% 15 3.65 x 10-3 total 215 - 15.3 - mean 71.7 56.3% 46 - 1 Genotypes not exclusive among events 105 Texas Tech University, Anthony J. Giordano, May 2015 Table 3.2. Estimates of jaguar abundance using the TIRM in capwire for each of three CR-NGS events at DCNP. CI = confidence interval; Na= estimate of ‘easy-to-capture’ individuals; Nb= estimate of ‘difficult-to-capture’ individuals; α = CR parameter. Event Abundance (N) 95% CI CI Width Na Nb α (alpha) 1 29 17-42 25 8 21 4.7917 2 45 26-59 33 10 35 5.8926 3 36 20-49 29 9 27 5.3737 106 Texas Tech University, Anthony J. Giordano, May 2015 Table 3.3. Estimates of effective sampling area and jaguar density at DCNP for three CR sampling events. Sampling Event ESA (km2) Jaguar Density [AR]* (D= per 100 km2, 95% CI) Jaguar Density [AR] (D= per individual, 95% CI) 7142 0.41/ 100 km2 1/ 244 km2 (0.23 – 0.59/ 100 km2) (1/435 km2 , 1/170 E1 km2) E2 6173 0.73/ 100 km2 1/137 km2 (0.42 – 0.96/ 100 km2) (1/238 km2, 1/104 km2) 0.52/ 100 km2 1/192 km2 (0.29 – 0.71/ 100 km2) (1/345 km2, 1/141 km2) E3 6947 107 Texas Tech University, Anthony J. Giordano, May 2015 CHAPTER V JAGUAR (PANTHERA ONCA) POPULATION GENETICS ACROSS A DRY CHACO-PANTANAL LANDSCAPE: ASSESSING GENE FLOW BETWEEN HIGH PRIORITY JAGUAR CONSERVATION STRONGHOLDS ABSTRACT The jaguar (Panthera onca) has been the subject of considerable rangewide conservation planning effort and attention. As Latin America’s largest felid and widest-ranging carnivore, jaguars require large connected areas of habitat to ensure their long-term viability. Among numerous proposed conservation units for jaguars considered priorities between Mexico and Argentina, it is unclear where if any functional connectivity exists. We examined the genetic diversity of jaguars in the Paraguayan Gran Chaco and evaluated for the presence of gene flow or population structure between jaguars in the Chaco and the adjacent Brazilian Pantanal. We found high genetic diversity (HE = 0.757; AN = 10.375) and evidence for weak to moderate population structure between jaguars in these two regions (FST = 0.071; GST = 0.041, DEST = 0.348; G’ST = 0.374; G”ST = 0.399; p<0.05). We also found evidence of moderate isolation-by-distance (IBD) effects (Mantel Tests, R2 = 0.2047 – 0.2280; p<0.05) consistent with the presence of clinal variation. An AMOVA indicated most molecular variation was accounted for within and among individuals, not between populations (FST = 0.113; p<0.05). STRUCTURE analyses not specifying prior population information produced no support for either K = 1 or 2, although the greatest second order rate of change of the likelihood distribution (ΔK) was from K = 1 to 2; analyses incorporating a priori geographical information suggested only marginal support for K=2 over K=1. While our results suggest the occurrence of gene flow between these two important regions and are consistent with a single panmictic population balanced by isolation by distance, some measures of genetic differentiation indicate evidence of population structure, possibly due to deforestation and habitat 108 Texas Tech University, Anthony J. Giordano, May 2015 fragmentation driven by expanding commercial cattle-ranching interests. We conclude that continued development and habitat loss across the Chaco-Pantanal landscape may jeopardize connectivity between these two high priority jaguar conservation strongholds, and recommend multi-national cooperative planning to prevent this. INTRODUCTION As a taxonomic group, mammalian carnivores typify the broad range of responses to changing anthropogenic land use patterns and modifications. While some carnivores benefit from human modifications to landscapes [1, 2, 3, 4], this is the exception rather than the rule for most species. Most large carnivores have relatively large home ranges and exist at low population densities, making them vulnerable to extensive habitat conversion and fragmentation, processes thatusually exacerbate direct conflict and competition with local people [5, 6, 7, 8, 9]. Often because of these same ecological attributes, carnivores are frequently focal species for broadscale conservation planning, as well as for assessing the efficacy and functionality of habitat movement corridors [10, 11, 12, 13, 14, 15]. In addition, their importance to overall ecosystem health and functioning, and their potential tofacilitate the maintenance of biodiversity previously has been established [16, 17,18,19, 20, 21,22, 23]. Jaguars and other large felids are typical of those solitary carnivores capable of long distance movements and dispersal [12, 24, 25,26, 27, 28], behavior which can theoretically facilitate high gene flow and connectivity across expansive landscapes. However, the quality and relative connectivity of the intervening habitat matrix often determines success or failure for individuals undertaking far-ranging movements or dispersal events [29, 30, 31,32, 33]. Cryptic and difficult to observe directly, jaguars have most frequently been studied through the use of camera-trapping [eg., 34, 35]. However, use of noninvasive genetic sampling (NGS) frameworks can now allow conservation planners to assess the genetic diversity and structure of populations in and among priority regions important to the long-term viability of jaguar populations. 109 Texas Tech University, Anthony J. Giordano, May 2015 This is important given that jaguar populations are declining due to habitat loss and its consequences [36], and currently occupy < 46% of their recent historical distribution [37]. Unlike the puma (Puma concolor), which exhibits considerable evidence of regional population structure across its range [38], jaguars exhibit high genetic diversity and little evidence of structure across their range [39]. However, Neotropical pumas appear to persist in degraded forest and among forest-pasture or agricultural mosaics where jaguars have already disappeared or are declining [40, 41, 42; 43, 44], suggesting they may be less vulnerable to large-scale landscape modifications than jaguars. Genetic evidence suggests that jaguars in the Upper Parana Atlantic Forest (UPAF) fragments of Brazil and Argentina are already effectively isolated from each other by a landscape matrixhostile to long-distance dispersal and movements [43]. Less well understood however is how a mosaic of rapid deforestation, pasture, and agricultural land might impact gene flow and connectivity among jaguars distributed across a landscape of otherwise contiguous natural habitat. One large geographical region considered important to the jaguar’s long-term future contains the Dry Gran Chaco and Pantanal biomes, where two stronghold jaguar conservation units (JCU’s)occur[37,45]. Spanning the trinational convergence of Paraguay, Bolivia, and Brazil, the Chaco-Pantanal landscape is important to a proposed rangewide network of functional connectivity among jaguar populations, and is critical to securing viable jaguar populations near the southern edge of their range. Though considered an area where jaguars have a “high probability of persistence” [37], this region has been experiencing rapid deforestation and land conversion for cattle-ranching in recent years [46, 47, 48, 49]. Also,given the relatively dense network of ‘corridors of concern’ (i.e., contiguous habitat corridors < 10 km in width) across south-central South America [45], the establishment of baseline data on connectivity among jaguars in this region represents an important need. Our goal was to investigate the genetic diversity and molecular variation in jaguars from the Paraguayan dry Chaco (Alto Paraguay). We compared and contrasted these results 110 Texas Tech University, Anthony J. Giordano, May 2015 with results from an analysis of a smaller sample of jaguar scats collected from the state of Mato Grosso do Sul in the Brazilian Pantanal. Finally, we evaluated for evidence of gene flow between jaguar populations in these adjacent ecological regions separated by the Rio Paraguay – one of the largest rivers in southern South America. STUDY AREA Most samples from Paraguay (Figure 1) originate from the western portion of the Gran Chaco Biosphere Reserve (GCBR) in the northern Dry Chaco. Samples from Brazil(Figure 1) originate from private land in the Pantanal state of Mato Grosso do Sol. At > 1 million km2 in historical total area [50, 51] the Gran Chaco includes the largest contiguous forested biome in the Neotropics outside of the Amazon Basin [51]. In Paraguay the Gran Chaco occupies approximately 60% of the country by land area, nearly all of it west of the Rio Paraguay. The western GCBR includes the driest parts of Paraguay and the Chaco, with annual precipitation totals ranging between 400 mm and 600 mm, most falling between October and March [52]. Median annual temperatures are 26-27°C. Dominant trees typical of the Dry Chaco region include the softwood ‘bottle trees’ such as Chorisia insignis and Brachychiton sterculia, as hardwood timbers like the white quebracho (Aspidosperma quebracho-blanco), coronillo (Schinopsis quebracho-colorado), red quebracho (Schinopsis lorentzii), and the verawood or ‘Palo Santo’ Tree (Bulnesia sarmientoi) [53, 54]. Temperatures are comparable in the Pantanal, a low elevation alluvial floodplain wetland constituting one-third of the Upper Paraguay River Basin and comprising an expansive ecotone with the Gran Chaco to the east and north of the latter. The Pantanal is one of the largest contiguous wetlands in the world and lowlands flood extensively during the rainy season (October – March), when as much as 80% of the annual 800 – 1600 mm of precipitation falls [46]. Though predominantly a Brazilian biome (80%), small portions of the ~210,000 km2 Pantanal extend into the eastern GCBR of northern Paraguay,as well as southeastern Bolivia, across the trinational border of the three countries. Intensification of cattle-ranching is leading to increased fragmentation and conversion of both the northern Dry Chaco and 111 Texas Tech University, Anthony J. Giordano, May 2015 Pantanal, and represents one of the dominant threats to biodiversity in both regions [46 47, 48,49]. METHODS Sample Collection& Species Identification We collected scat samples of jaguars across broad parts of public and private land in the northern Paraguayan Chaco (Alto Paraguay), and private land in the Brazilian Pantanal (Mato Grosso do Sol). One additional sample from Paraguay originated from Paso Bravo National Park in the cerrado ecoregion east of the Rio Paraguay. Samples from the Paraguayan Chaco were collected in their entirety and stored until processed; a small portion of each scat encountered in Brazil was collected and stored in DMSO (DET) buffer until analysis. The processing of all Chaco scat samples to confirm their jaguar origin is described in detail by Giordano et al. [55]. DNA extraction methods to confirm species identity followed slight modifications to the protocol provided by Qiagen ® (Stool Sample DNA Extraction Kit, #51504) [55]. Optimization and reaction profiles for PCR amplification followed modifications to Haag et al. [56] for the ATP-6 region of mtDNA; the final elute for each sample was purified using an ExoSAP solution before being sequenced at the University of Arizona’s Genetics Core Facility (3730 Automated DNA Analyzer; Applied Biosystems, Foster City, CA, USA). To determine species origin, all sample sequences were aligned using Sequencher ® (Gene Codes Corporation, Inc.) and compared to ATP-6 reference sequences [56] using the BLAST partial match nucleotide sequence algorithm [57] in the NCBI GenBank Database [58]. Genotyping To identify individuals, we used a subset of eight polymorphic microsatellite loci (FCA77, FCA126, FCA161, FCA211, FCA229, FCA441, FCA453, FCA678) of a 112 Texas Tech University, Anthony J. Giordano, May 2015 panel of 29 originally identified for the domestic cat [59]but used successfully in prior genetic studies on captive and wild jaguars [39, 43, 56]. PCR amplification occurred in a total per sample volume of 10 μl with the following component concentration: 3.5 μl of distilled H2O, 1.0 μl of PCR Buffer 10X (Qiagen, Inc.), 0.4 μl of 25 mM MgCl2 (Qiagen, Inc.),0.25 μl of 10mM dNTPs (Qiagen), 0.25 μl of 1% BSA (Sigma-Aldrich, Inc.),0.5 μl of M13-tailed forward primer (1 μM), 0.5 ul of M13-tailed reverse primer (10 μM), 0.5 ul of VIC (green) Dye (10 μM) (Qiagen, Inc.), 0.1 U of HotStarTaq ® (Qiagen, Inc.) at 5 U/μl, and 3 μl of undiluted template DNA of unknown concentration.All amplifications were performed using PCR Thermocycler machines (Mastercycler ®, Eppendorf, Westbury, NY, USA). The reaction profile for each sample consisted of an initial denaturation of 3 minutes at 95°C, followed by 40 Touchdown cycles of (1) 95°C of 15 seconds (denaturation), (2) 55°C for 15 seconds (annealing), and (3) 72°C for 30 seconds. A final extension step at 72°C for 30 minutes concluded the reaction and samples were held at 4°C until removed. To improve amplification success of degraded DNA samples and reduce possible noise in scoring allele peaks of potentially overlapping fragment lengths, all microsatellite loci were uniplexed (i.e., amplified individually) using a single fluorescent dye (VIC; GelPilot 5x, Qiagen, Inc) and subjected to three independent PCR attempts. Allele peaks for all samples were scored independently by three observers in GENEMARKER ® (SoftGenetics, State College, PA) and compared to positive controls obtained from known jaguars (eg., white blood cells, fresh scat). We used GIMLET v1.3.3 [60] to determine consensus genotypes and estimate our genotyping error rate across all loci and PCR attempts for all samples, calculate PID(sib) values for each locus and overall for all samples[61, 62], and exclude potentially redundant (i.e., matching) samples. Due to the smaller number of samples from Brazil and lower amplification rates across all eight loci, we included confirmed jaguar samples where at least four loci successfully amplified. Samples from Paraguay were included only where at least seven loci successfully amplified. 113 Texas Tech University, Anthony J. Giordano, May 2015 Genetic Diversity & Variation Deviations from the assumption of Hardy-Weinberg Equilibrium (HWE) were assessed using both GENEPOP 4.2.1 [63, 64, 65] andFSTAT 2.9.3 [66, 67]. We used the complete enumeration method to calculate exact test P-values (for loci with < 4 alleles) [68] and an unbiased estimation of the exact HWE probability (for loci with > 4 alleles)[69] using a Markov chain process (dememorization of 10000; 1000 batches; 5000 iterations/ batch). We tested for linkage disequilibrium (LD) by examining all pairwise comparisons of loci in GENEPOP at a significance threshold of α < 0.05 using the default settings. The significance of p-values for HWE and LD was adjusted for multiple comparisons using the Bonferroni correction [70]. We used FSTAT and GENALEX 6.5 [71] to calculate measures of genetic diversity, including the number of alleles (AN) detected at each locus, the mean number of alleles across all loci, the observed (Ho) and expected (He) heterozygosity, and the Shannon’s ‘Diversity’ Information Content (I), for jaguars from the Paraguayan Chaco and the Brazilian Pantanal. We estimated allelic richness (AR) [cf. 72] using programs FSTAT and HP-Rare, and the number of unique or private alleles for Chaco and Pantanal jaguars using the program HP-Rare, which accounts for unequal samples sizes [73, 74]. Genetic Differentiation & Isolation By Distance As there is little consensus regarding approaches to measure genetic differentiation, we used several to assess gene flow between the Gran Chaco and Pantanal. We calculated pairwise multilocus estimates of FST and related F-statistics [cf. 75, 76, 77] and pairwise estimates of RST [78,79] using several programs, including GENEPOP, F-STAT, and GENALEX. The significance of all p-values was tested using the genic differentiation test (GENEPOP) and adjusted for multiple comparisons using a Bonferroni sequential correction [70]. However, because microsatellites have the potential to bias traditional FST calculations due to their highly polymorphic nature [80] making traditional measures of differentiation alone inadequate [81], we usedSMOGD 2.6 [82] and GENALEX 6.5 [71] to calculate 114 Texas Tech University, Anthony J. Giordano, May 2015 standardized measurements of differentiation, and those corrected for fewer (eg., 2) populations (K). This includes: GST (corrected for small population and inbreeding [cf. 83,84], Hedrick’s standardized (0 to 1) G’ST [85],Nei’s standardized G’ST correcting for small k [86], Jost’s DEST [80,87], and G’’ST, an alternative standardized measurement of Hedrick’s GST further corrected for a low number of populations (i.e., K) [86]. We also conducted an analysis of molecular variation (AMOVA) in GENALEX to examine how the genetic variation we observed was partitioned within and among individuals and populations [88]. To evaluate for overall isolation by distance (IBD) effects [89, 90] among jaguar genotypes, we conducteda nonparametric Mantel permutation test [65, 91] following Smouse et al. [92] (999 permutations) to evaluate the null hypothesis of independence between the genotypic distance of microsatellite (codominant) data [76, 93] and geographic data. Geographical distance vectors were determined by calculating the Euclidean distance between the centers of sample clusters (populations); genetic distance was expressed as FST/(1 – FST) and formed the basis for creating the genotypic distance matrix. We also conducted spatial structure analyses of genotype and geographical distances assuming a single population for all of our samples [94]. We did this in GENALEX using 30 Even Distance Classes (EDC) of 20, 28 Even Sample Classes (ESC), and multiple distance classes (MDC) (20 runs, Based Distance Class = 10), which evaluates the effect of the number of samples in distance classes on the correlation coefficient (999 Permutations, 1000 Bootstraps for EDC, ESC, and MDC). As a final measure of IBD, we conducted a two-dimensional local spatial autocorrelation analysis (999 permutations, 1-tail probability test, 10 Nearest Neighbors, cutoff p<0.05) for each individual genotype against geographical distance [71]. To assess relative genetic connectivity between populations, we conducted population assignment tests using model-based clustering in program STRUCTURE 2.3.4 [95]. Analyses were performed across 10 iterations of 500,000 Markov Chain Monte Carlo (MCMC) repetitions and a burn-in period of 100,000; all allele 115 Texas Tech University, Anthony J. Giordano, May 2015 frequencies were assumed to be independent (i.e., “unlinked”) [95]. To assign individuals to one of K clusters, we used the admixture modelfor ‘correlated allelic frequencies’ for each independent iteration of K = 1-10 (100 iterations total). To determine the optimum number of clusters (K) and contrast the proportional explanatory power of all values for K = 1-10,we compared both ad hoc estimates of the optimal K (i.e., K = X; the K most representative of the data) by plotting -ln Pr(X|K) over K [95,96,97], and also using the greatest ΔK (the second order rates of change of ln[Pr(X|K)) via STRUCTURE HARVESTER [98]. We did this assuming three scenarios: (1) a priori specification of population origin for each sample, (2) no apriori specification of each sample as to the population origin, and (3) no apriori specification of population origin from only the Paraguayan Chaco. Bayesian genetic clusters based on sample genotypes were compared to the geographical locations of samples to designate population origins; each group was tested for both HWE and linkage equilibrium with a Bonferroni correction to account for locus-population interactions [95,97]. Mean population assignment probabilities (q) of all iterations for the optimum K value were calculated using the ‘Full Search’ algorithm weighted by the number of individuals in CLUMPP [99] for each of the three scenarios outlined above. RESULTS PCR amplification success for all 8 loci were higher for samples collected from the Paraguayan Chaco and stored dry (40%, n=264) than those samples collected from the Brazilian Pantanal and stored in DMSO (15%, n=33). Estimates of allelic dropout across all samples were slightly higher for samples from the Gran Chaco (0.0845) than those from the Pantanal (0.0570). We recorded no evidence of null or false alleles in our dataset. Probability of identity estimates for the pooled (global) population (P(ID)sib = 5.215 x 10-5), the Paraguay population (P(ID)sib = 6.290 x 10-4), and the Brazil population (P(ID)sib = 5.255 x 10-4), were sufficient to resolve individuals in our sampled populations (P(ID)sib< 0.001) [62]. We identified 40 unique individuals from Paraguay where >7 (7-8) loci were successfully genotyped, and ten individuals 116 Texas Tech University, Anthony J. Giordano, May 2015 among samples from Brazil where >4 (4-8) loci amplified. Despite the smaller sample size from Brazil, differences among them were sufficient to confidently assign samples to different individuals (i.e., genotypes differed at >2 loci). We initially detected linkage disequilibrium (LD) for seven pairs of loci at α = 0.05 (uncorrected), including four involving FCA77 (FCA161, FCA211, FCA 453, FCA 678), two involving FCA 678 (FCA161, FCA211), and between FCA161 and FCA 453. However, after an adjusted Bonferroni correction (p<0.001786), we detected no significant evidence of linkage disequilibrium and therefore retained all loci for subsequent analyses. Similarly, one locus (FCA441) for jaguars from the Paraguayan Chaco was considered out of HWE with four loci (FCA126, FCA161, FCA211, and FCA441) at the unadjusted α = 0.05 level, but no evidence of deviation remained after Bonferroni correction (p<0.00313). Genetic Diversity & Variation Per locus Observed (HO) and Expected (HE) Heterogzygosity ranged from 0.606 – 0.919, and 0.662 – 0.829, respectively for jaguars from the Paraguayan Chaco (Table 1). The pooled mean HO and HE for both populations was 0.623 and 0.757 respectively, with both measures more similar for the population of Chaco jaguars (HO = 0.723, HE = 0.733) than for those from Brazil (HO = 0.523, HE = 0.780). This is likely due to the greater sample size from Paraguay and greater overall PCR amplification success across all loci (Table 2). Similarly, a mean of 2.625 more alleles per locus were recorded from Paraguayan jaguars than jaguars from Brazil. The mean number of observed alleles (AN) across all 8 loci was 10.375 for the pooled population, and the mean Shannon-Weaver Information Index (I) of allelic diversity was similar for both populations (Table 2). Our estimate of HE for Pantanal jaguars was slightly greater than for the Chaco, a measure that was consistent with other comparative measures of genetic diversity between the two populations. Allelic Richness (AR) of jaguars (4.44) from the Brazilian Pantanal (4.77) was larger than for the Chaco (4.08). Interestingly, despite the larger mean number of private alleles per 117 Texas Tech University, Anthony J. Giordano, May 2015 locus recorded (PAO) from the Chaco (4.375) relative to the Pantanal (1.750), not unexpected given sample size differences, a rarefaction-based estimate of mean private allele richness (PAE) using a minimum of 8 alleles (ie., the minimum number of alleles at any locus for the pooled population) was 1.33:1 for Pantanal to Chaco jaguars, suggesting that additional private alleles in the Pantanal population remained to be sampled. Population Differentiation &Structure Estimates of FST between both populations as calculated in GENEPOP (0.088) and GENALEX (0.071) weren’t large, though values were significant (p<0.001) overall for all loci. The mean overall weighted RST value for all loci as calculated in GENEPOP and F-STAT was approximately 0.22. Overall, values for the three standardized measurements of differentiation (Hedrick’s G’ST, Jost’s DEST, and G’’ST) were similar (0.348 – 0.399) and comparable for all 8 loci (Table 3), suggesting they behaved very similarly. In addition, all measures of differentiation between population pairs (GST, Nei’s G’ST, Hedrick’s G’ST, G”ST, DEST) were significant overall (p<0.001) (Table 3). Among all loci, only locus FCA126 wasn’t significant for five measures (p>0.05), though it was nearly significant for Hedrick’s G’ST (p=0.059) and Jost’s DEST (p=0.056). Analysis of Molecular Variation (AMOVA) using genetic distance matrices indicated a slightly greater overall pairwise FST value (0.116; p<0.001) and resulted in an estimate of 1.9 effective migrants (Nm) per generation. That the AMOVA-based FST is slightly larger than the maximum traditional FST (G-statistic) isn’t surprising given the latter is inversely proportional to the number of alleles [100]. Hierarchical partitioning of genetic variation indicated that the great majority of variation observed in the data (68.1%) can be explained by the genetic diversity within individuals. Much less (20.3%) variation was due to genetic differences among individuals, while the remainder (ie., AMOVA FST = 11.6%) was due to population differences. The AMOVA-based RST (0.306, p<0.001) [78, 79] suggested that between population 118 Texas Tech University, Anthony J. Giordano, May 2015 differences (30.6%) accounted for less of the observed variation than variation among individuals (68.7%). We detected evidence for moderate Isolation-By-Distance (IBD) effects among the jaguars we sampled. Linear geographical and log-transformed geographical Mantel tests examining the relationship between Nei’s genetic distance matrix [93] for all individuals resulted in comparable coefficients of determination (R2 = 0.2280, R2 = 0.2047)and indicated a highly significant relationship (p<0.001) (Figure 2a,b). In addition, c spatial structure analysis using expanding multiple distance classes [71, 100] show r-coefficients stabilize at approximately 0.015 – 0.016 and indicated a weakly significant (no C.I.’s overlapped 0 for all permutations) autocorrelation between increasingly larger distance classes, and the correlation coefficient for each of those distance classes (Figure 3). Spatial structure analysis using both 30 even distance classes (EDCs) and 30 even sample classes (ESC’s), where confidence intervals for 9 of 30 distance classes, and 9 of 28 distance classes, respectively, were significant (ie., didn’t overlap 0) over 1000 bootstraps. Results of a two-dimensional local spatial autocorrelation (2D LSA) analysis suggested that 21 of the 50 individual jaguars exhibited a significant relationship (p<0.05) between genetic and geographical distance, including 7 of the 10 jaguars from Brazil, although effect sizes were not large for any of these individuals (0.0391 - 0.136). Interestingly, two samples from Paraguay collected further away than the others did not appear to exhibit this relationship. When incorporatinga priori information regarding each sample’s population origins, the highest mean likelihood value occurred for K = 2 (Ln P(K) = -1280.85; SD = 46.32) among all runs of K = 1-10. The only positive mean rate of change for the likelihood distribution (|Ln’(K)| = 15.79) was also for K = 2. However, the mean likelihood for K = 1 was only slightly lower (-1296.64) and was accompanied by much greater precision (SD = 0.41). In addition, the largest ΔK value ([|Mean(L”(K)|/SD(L(K)) occurred for K = 1 to 2, lending additional support to K = 2 as the optimal K. Evidence of some admixture with jaguars from the Pantanal 119 Texas Tech University, Anthony J. Giordano, May 2015 population was clearly evident in Chaco jaguars, with mean (10 runs of K =2) population probability assignments (q) ranging between 0.67 – 0.98 (Mean across all individuals, q = 0.92) and suggestive of low levels of gene flow between jaguars in both regions (Figure 4). Only 4 of the 40 samples had mean population assignment probabilities of q< 0.9 (Figure 4) however, which is not inconsistent with an equilibrium maintained by isolation-by-distance. Interestingly, while seven of the ten individuals from the Pantanal exhibited a high probability they were from that population (Mean q = 0.87 – 0.98), three individuals appeared to originate from the Gran Chaco (Mean q = 0.79 – 0.86), or were descended from recent migrants that emigrated from the Gran Chaco. For analyses where no prior population assignment was indicated in STRUCTURE, the lowest mean likelihood value of all values for K = 1-7 (Ln P(K) = 1296.69) was for K =1; only mean likelihood values for K = 8 (-1325.98), K = 9 (1323.73), and K = 10 (-1337.78) were lower overall. The highest mean likelihood value was for K = 5 (Ln P(K) = -1200.51), followed by K = 3 (-1241.70) and K = 2 (1243.21). Precision for all 10 runs of K = 2 (0.91) was greater than for all other values of K = 2 – 10, as was the mean rate of change (|Ln’(K)|) for the likelihood distribution (53.48). The ΔK ([|Mean(L”(K)|/SD(L(K)) was greatest for K = 1 to 2 (57.36; Figure 5), a five-fold greater value than for K = 4 to 5 (11.41), and 30x and 100x greater than K = 3 to 4 (1.93), and K = 2 to 3 (0.56), respectively. Graphic representation of population assignment probabilities for 10 runs of K = 2 suggested high levels of admixture in jaguars from the Gran Chaco (Figure 6), as STRUCTURE’s Bayesian clustering algorithms could not appear to consistently identify samples from this region as being from the Chaco population. Finally, a STRUCTURE analysis of only jaguars from the Paraguayan Chaco without use of prior information yielded the highest mean likelihood value (Ln P(K) = -991.14) and smallest standard deviation for (1.06) for K = 1. By definition ΔK cannot be computed for K = 0 to 1, as support for “no population” (K = 0) cannot be calculated. However, the remaining ΔK values spanned a small interval for all K = 2 120 Texas Tech University, Anthony J. Giordano, May 2015 to 10 (0.44 to 7.71), not inconsistent with jaguars in the Paraguayan Chaco representing a single population. The lack of agreement among support for the optimum number of clusters based on the greatest ΔK value (7.71 for K = 2 to 3; support for K =3), the highest mean rate of change (|Ln’(K)| = 64.77; support for K = 4), and the greatest absolute value of the 2nd order rate of change of the likelihood distribution ((|Ln”(K)| =359.85; support for K = 7) is consistent with jaguars in the Paraguayan Chaco being highly admixed, as none of these “population” scenarios has sufficient biological basis. DISCUSSION Large carnivore species around the world are exhibiting substantial range collapse and population declines [5,9, 101]. Fragmentation and habitat conversion, and the resultant increase in direct human conflict and decrease in prey availability, remain the greatest threat to the persistence of many carnivores around the world (1,5, 6, 9, 102]. In recent years, big picture planning efforts have become increasingly effective conservation tools for large carnivore species across extensive geographical regions (37, 103, 104,105), and rangewide connectivity of jaguar habitat across Latin America has received considerable attention in this regard [37,45]. Although most evidence suggests jaguars exhibit little genetic structure across their range [39], few regional studies have sought to directly assess the fine-scale regional genetic relationships among jaguars [eg., 43]. Our study is the first investigation to examine the genetic relationship among jaguars in adjacent ecological regions and JCU’s. It is also the first to investigate genetic diversity and connectivity among jaguars across a large contiguous regional landscape. Genetic Diversity & Variation Jaguars have been characterized as exhibiting high microsatellite diversity across their range. This is consistent with little geographical isolation to accrue sufficient differentiation among regional populations, the maintenance of high gene flow and the ability to disperse across great distances, a relatively recent population 121 Texas Tech University, Anthony J. Giordano, May 2015 expansion, and high molecular variation within or among individuals rather than among populations [39]. Our results suggest that the genetic variation of jaguar populations in the Gran Chaco and Pantanal is consistent with the few prior examinations of jaguar conservation genetics. Consistent with prior research, we detected much greater genetic variation within and among individuals than between our two proposed populations. In addition, our measurements of overall genetic diversity are consistent with prior findings. Eizirik et al. [39] reported an average of 8.31 alleles for jaguars across their range, slightly fewer than our 10.38; however, their mean included 29 loci compared to our eight. The observed number of alleles in the UPAF of Brazil and northern Argentina was fewer among four forest fragment sites (7.2), possibly owing to the impacts of genetic drift [43]. Our allelic size range for all 8 loci (22.75 bp) was much greater than that recorded by Eizirik et al. [39] (9.8 bp). Ruiz-Garcia et al [106] also recorded a great allelic size range (>20 bp) in jaguars for 9 of 12 of the original Menotti-Raymond et al. [59] loci, including the same 16 bp range for FCA126 we detected, the only locus our studies had in common. For pumas in southeastern Brazil, an average allelic range of 24.3 was recorded across seven microsatellite loci[107]. Expected heterozygosity (HE) was comparable among jaguars from the Paraguayan Chaco (0.733), those from the UPAF (0.732) [43], and the rangewide (0.739) and southern population (0.724) of jaguars [39], despite differences among studies with respect to the loci examined overall. Observed heterozygosity (HO) was slightly higher in the Chaco (0.723) than for the UPAF (0.682), possibly due to the effects of drift in the latter. That HO (0.523) and the observed number of alleles (6) was smaller for jaguars from the Pantanal may have been due to the much smaller number of samples we had from the latter, as HE in the Brazilian Pantanal (0.780) is high for a single region. Other measures of genetic diversity, including allelic richness and the estimated number of private alleles (Table 2), also suggested that the Pantanal population was more diverse. A prior study that examined a high number of samples from Columbia (n = 62), as well as 22 additional samples from six other countries, 122 Texas Tech University, Anthony J. Giordano, May 2015 recorded higher diversity values for jaguars (HE = 0.846, Mean AN = 11.33/ locus for 12 microsatellite loci) overall [106]. Population Differentiation & Structure Our FST values ranged from 0.071 to 0.116, indicative of gene flow and at most, weak differentiation, between jaguars in the Gran Chaco and the Pantanal. Suggestions of what thresholds values represent differentiation vary and are somewhat subjective; significant biological differentiation for example has been proposed as beginning at FST = 0.15 [108], whereas values ranging from 0.05 to 0.15 have been characterized as moderately differentiated [109]. Using many more loci though with little regional representation, a distribution wide analysis suggested three to four incompletely isolated phylogeographic partitions of jaguars corresponding to northern South America, south of the Amazon River, and possibly two partitions within Central America (FST = 0.065) [39]. While the overall FST value among multiple UPAF fragments was comparable to ours for the two regions (FST = 0.089), the FST valuebetween the two most differentiated populations was relatively larger (0.198), consistent with the greater isolation of jaguars that might be expected under this scenario [43]. We report an RST of 0.224, greater than for the rangewide analysis (0.183) [39] and much larger than for the UPAF (RST = 0.075) [43]. Haag et al. [43] suggested this low RST : FST ratio was consistent with a more prominent role for genetic drift in the process of differentiation. We recorded the opposite, ie., a relatively high ratio of RST : FST, which accordingly may be consistent with genetic drift being less important in explaining in the differentiation we observed. Although RST was originally developed for microsatellites to compensate for their variation in allelic size range [79], care must be taken in interpreting conclusions based on RST, as assumptions regarding simple step-wise mutations upon which these calculations are based are rarely valid for natural populations [100, 110]. 123 Texas Tech University, Anthony J. Giordano, May 2015 In contrast to our FST values, which suggested low levels of differentiation, our standardized measurements of differentiation were somewhat higher, suggesting moderate differentiation between jaguars in the Gran Chaco and the Pantanal. In fact, the strongest evidence for genetic differences between these two populations was provided by the relative consistency in the performance of our standardized G’ST values. Taken together, they suggested that differences between the two populations occurred at approximately 35-40% of the maximum level possible given the observed genetic variation in the data (eg., shared alleles, allele frequencies, etc.)[85]. These values are noteworthy because traditional FST values suffer from limitations, or a negative bias, when using highly polymorphic markers such as microsatellites. Because biallelic (not multi-allelic) markers were the original basis for FST values, larger numbers of alleles for loci ultimately limit the maximum possible value for FST [81, 85, 111]. Jost’s D (DEST) values for UPAF fragments exhibited great range across pairwise site comparisons (0.052 – 0.313) [43], smaller than our overall DEST estimate. However unlike us, they concluded that genetic drift played a role in differentiation among their populations, which can be expected to result in a decline in allelic diversity, and thus a decrease in the maximum value of Jost’s D. Evidence for moderate Isolation-by-Distance (IBD) effects among our samples suggest an equilibrium of relatively low or restrictedgene flow. Approximately 40% of our samples exhibited significant spatial autocorrelation with genetic distance, including seven of the ten samples from the Pantanal. Not surprisingly, population assignment probabilities for these seven Pantanal jaguars suggest low levels of admixture from the Gran Chaco. In contrast, the lower percentage of individuals from Paraguay (35%) exhibiting spatial autocorrelation with genetic distance is consistent with greater gene flow from the Brazilian Pantanal to the Dry Chaco. The results of our STRUCTURE analyses further lend support to this idea, as they demonstrate high admixture among jaguars from the Chaco. That gene flow may occur predominantly from the Pantanal to the Paraguayan Chaco is consistent with jaguars moving from an area of relatively high population density [24, 112] to one where density estimates are 124 Texas Tech University, Anthony J. Giordano, May 2015 among the lowest reported from across their range [26]. Substantial molecular variation both within and among individuals has been observed for jaguars across their range, characteristics consistent with the long-distance dispersal and colonization ability of male jaguars, and IBD [39]. Our AMOVA-based estimate of the effective number of generational migrants (Nm = 1.9) are low and not inconsistent with IBD. Although this exceeds the classical one-migrant-per generation rule, a threshold originally proposed as adequate to maintain gene flow and prevent differentiation [113,114, 115], it is much lower than the 10 migrants per generation that other, more recent studies have proposed as needed to prevent genetic differentiation [115, 116] Use of Nm as a measure of gene flow for jaguars however may be confounded by their recent, rapid colonization of South America [39], and it is unclear how much practical value this measure has for the species. Aside from IBD effects, other factors could potentially account for restricted gene flow between these two regions. Given their relative proximity, it is almost a certainty that broadscale habitat connectivity existed between the Gran Chaco and Pantanal, and a broad transition zone between the two is still evidenced in the eastern part of far northern Paraguay [46, 117]. However, these two ecological regions may represent contrasting extremes in habitat, a factor of potentially important consideration for non-resident and dispersing individual jaguars in search of a vacant new territory. The Rio Paraguay also separates both ecological regions, and could slow gene flow from the Pantanal. Riparian gallery forests of the Rio Parana, another major regional drainage system, actually facilitated jaguar movements among isolated UPAF fragments, suggesting this river was far from a barrier [12]. Also, movement data for two female jaguars monitored in the northern Paraguayan Pantanal [26] indicated both animals had little difficulty repeatedly crossing the Rio Paraguay. The Amazon River wasn’t ultimately an impediment to the historical southern movements of jaguars into South America, despite evidence of incomplete geographical partitioning [39]. Still, evidence of incomplete geographical partitioning among the continent’s jaguars was most associated with the Amazon River largely due to the 125 Texas Tech University, Anthony J. Giordano, May 2015 differential impact it had on the movement of males and females [39], the latter of which we conclude were more restricted by the River. Perhaps of greatest significance is a recent history of rapid, intensive land use change and habitat conversion across the Pantanal [46, 47]and the Gran Chaco [48,49], which may be contributing to some of the population structure we observed. Substantial agricultural development now exists across the region, including across the expansive Chaco-Pantanal transitional zone across the borders of Paraguay, Bolivia, and Brazil, particularly between the two populations (Figure 6a, b). Increased emphasis on cattle production due to rising beef prices has over several decades resulted in substantial fragmentation of Chaco, Chiquitano, and riparian gallery forests, and natural watercourses, across much of the Upper Rio Paraguay Watershed Basin [46, 47,48, 49, 118]. These processes may be exacerbating conflict with landowners, which together may now be restricting gene flow and dispersal between the two regions. Such processes may also be disrupting social structure and behavior among jaguars in the Gran Chaco, lowering demographic saturation thresholds and possibly impacting dispersal patterns. STRUCTURE population assignment probabilities for three Pantanal jaguars (q = 0.79 – 86) are either consistent with their Chaco origins or recent ancestry, and suggest some recent gene flow in the opposite direction. Giventhat jaguars in the Gran Chaco are known to range over particularly large areas (Mean HR = 410 km2, 95% kernel; n=6), even relative to other habitats [26], this is certainly feasible. Demographic saturation has also been proposed for jaguars in the UPAF [43], which are forced to leave existing forest fragments and wander unsuitable habitat in search of new territories. Jaguars have been recorded as moving 30 km in 3-4 days in this landscape [12]. Two young adult females in one isolated UPAF fragment were determined to be recent migrants that had traveledacross many kilometers of unsuitable habitat [43]. 126 Texas Tech University, Anthony J. Giordano, May 2015 Conservation Implications The genetic pattern we observed for jaguars across the Gran Chaco and Pantanal suggests that limited gene flow is occurring between jaguars in these two regions, but that moderate levels of differentiation are present. However, while we believe that IBD effects, and a lack of adequate geographical representation across a hypothetical cline, may have influenced the differences we observed, they may not be responsible for all of it. Many studies have already demonstrated that jaguars can disperse over long distances, through diverse habitats and landscapes, across rivers, and over periods of several months [12, 24, 39, 119], suggesting that these factors are not barriers to jaguar movements. Instead, a recent geographical history of rapid and intense deforestation, intensification of agriculture and livestock ranching, and expanding human-jaguar conflict [47, 49, 120, 121, 122, 123], may be more important factors in limiting jaguar gene flow, and continue to present the greatest threats to the region. In contrast to the discrete, isolated fragments of UPAF [43], the Gran Chaco and Pantanal ecoregions merge in an extensive mosaic of landscape transformation, seasonal flooding, and habitat of varying suitability to jaguars. In addition, the two regions are different ecologically and may represent extremes in jaguar density,potentially compounding the challenges to regional jaguar conservation planning efforts. Despite these challenges, connectivity of jaguar populations and successful dispersal can effectively occur in the context of an effective threat mitigation framework [12, 37, 45]. While the design and establishment of additional protected areas of adequate size, location, and spatial arrangement should be part of any comprehensive regional conservation strategy linking these two important Jaguar Conservation Units, a greater emphasis on local and cooperative conservation measures across the Gran Chaco and Pantanal is urgently needed. This includes trinational cooperation among the national and municipal governments of Paraguay, Brazil, and Bolivia. Greater cooperative transboundary monitoring of jaguar populations across these borders, and research on jaguar dispersal and mortality, 127 Texas Tech University, Anthony J. Giordano, May 2015 should be conducted to identify particularly problematic areas, including those zones of highest conflict between jaguars and local stakeholders. Similar efforts might do well to evaluate corridor characteristics and functionality with respect to jaguar movements to better assess their relative use, particularly ‘corridors of concern’ [45]. Campaigns aimed at increasing landowner awareness of jaguar conservation issues, and landowner support networks actively assisting with conflict resolution and forest conservation on private land, may be among the most effective solutions for quickly reducing local jaguar mortality. Enforcement of existing forest and wildlife laws in all three countries, even in remote areas far from principal municipalities, is also needed, possibly by incorporating the use of real-time remote imagery technology. Only a multi-faceted approach implemented with the cooperation and support of all private and public stakeholders will be effective in achieving jaguar conservation across this large, ecologically and socio-politically diverse landscape. 128 Texas Tech University, Anthony J. Giordano, May 2015 REFERENCES 1. Crooks K. Relative sensitivities of mammalian carnivores to habitat fragmentation. Conserv Biol. 2002;16:488-502. 2. Lyra-Jorge MC, Ciocheti G, Pivello VR. Carnivore mammals in a fragmented landscape in northeast of Sao Paulo State, Brazil. Biodivers Conserv. 2008;17:1573-1580. 3. Blaum N, Rossmanith E, Schwager M, Jeltsch F. Responses of mammalian carnivores to land use in arid savanna rangelands. Basic Appl Ecol. 2007;8:552-564. 4. Gehrt SD, Riley SPD, Cypher BL. Urban Carnivores: ecology, conflict, and conservation. 1st ed. Baltimore, MD: Johns Hopkins University Press; 2010. 5. Gittleman JL, Funk SM, MacDonald D, Wayne RK. Carnivore Conservation. Cambridge, UK: Cambridge University Press; 2001. 6. Treves A, Karanth KU. Human Carnivore Conflict and Perspectives on Carnivore Management Worldwide. Conserv Biol. 2003;17:1491-1499. 7. Cardillo M, Purvis A, Sechrest W, Gittleman JL, Bielby J, Mace GM. Human population density and extinction risk in the world’s carnivores. PLOS Biol. 2004;2:909-914. 8. Karanth, KU, Chellam R. Carnivore conservation at the crossroads. Oryx 2009;43:1-2. 9. Ripple WJ, Estes JA, Beschta RL, Wilmers CC, Ritchie EG, Hebblewhite M, Berger J, Elmhagen B, Letnic M, Nelson MP, Schmitz OJ, Smith DW, Wallach AD, Wirsing AJ. Status and ecological effects of the world’s largest carnivores. Science 2014;343:1241484. 10. Simberloff D. Flagships, umbrellas, and keystones: Is single species management passe in the landscape era? Biol Conserv.1998;83:247-257. 11. Carroll C, Noss RF, Paquet PC, Schumaker NH. Use of population growth viability analysis and reserve selection algorithms in regional conservation plans. Ecol Appl.2003;13:1773-1789. 129 Texas Tech University, Anthony J. Giordano, May 2015 12. Cullen L Jr. Jaguars as landscape detectives for the conservation of Atlantic forests in Brazil. University of Kent. 2006. 13. Ray J, Redford KH, Steneck R, Berger J. 2005. Large carnivores and the conservation of biodiversity. Island Press, Washington, D.C. 14. Rayfield B, Moilanen A, FortinM-J. Incorporating consumer-resource spatial interactions in reserve design. Ecol Model. 2009;220:725-733. 15. Parks SA, McKelvey KS, Schwartz MK. Effects of weighting schemes on the identification of wildlife corridors generated with least-cost methods. Conserv Biol. 2012;27:145-154. 16. Terborgh J. The big things that run the world. Conservation Biology 1998;2:402403. 17. Miller B, Dugelby B, Foreman D, Del Rio CM, Noss R, Phillips M, Reading R, Soule ME, Terborgh J, Willcox L. The importance of large carnivores to healthy ecosystems. Endangered Species Up.2001;18:202-210. 18. Ripple WJ, Larsen EJ, Renkin RA, Smith DW. Trophic cascades among wolves, elk, and aspen on Yellowstone National Park’s northern range. Biol Conserv.2001;102:227-234. 19. Terborgh J, Lopez L, Nunez P, Rao M, Shahabuddin G, Orihuela G, Riveros M, Ascanio R, Adler GH, Labert TD, Balbas L. Ecological meltdown in predatorfree forest fragments. Science 2001; 294:1923-1926. 20. Dalerum F, Somers MJ, Kunkel KE, Cameron EZ. The potential for large carnivores to act as biodiversity surrogates in southern Africa. Biodivers Conserv2008;17:2939-2949. 21. Dalerum F, Cameron EZ, Kunkel K, Somers MJ. Diversity and depletions in continental carnivore guilds: implications for prioritizing global carnivore conservation. Biol Letters2009;5:35-38. 22. Sergio F, Caro T, Brown D, Clucas B, Hunter J, Ketchum J, McHugh K, Hiraldo F. Top predators as conservation tools: ecological rationale, assumptions, and efficacy. Annu Rev Ecol Evol S. 2008;39:1-19. 130 Texas Tech University, Anthony J. Giordano, May 2015 23. Estes JA, Terborgh J, Brashares JS, Power ME, Berger J, Bond WJ, Carpenter SR, Essington TE, Holt RD, Jackson JBC, Marquis RJ, Oksanen L, Oksanen T, Paine RT, Pikitch EK, Ripple WJ, Sandin SA, Scheffer M, Schoener TW, Shurin JB, Sinclair ARE, Soule ME, Virtanen R, Wardle DA. Trophic downgrading of planet Earth. Science 2011;333:301-306. 24. Crawshaw PG Jr, Quigley HB. Jaguar spacing, activity, and habitat use in a seasonally flooded environment in Brazil. J Zool.1991;223:357-370. 25. Cavalcanti SMC, Gese EM. Spatial ecology and social interactions of jaguars (Panthera onca) in the southern Pantanal, Brazil. J Mammal.2009;90:935-945. 26. Giordano AJ, et al. [Chapter 4]. Abundance and density of the jaguar (Panthera onca) in the Paraguayan Dry Chaco: Use of a noninvasive genetic sampling framework. 27. Thompson DJ, Jenks JA. Long-distance dispersal by a subadult male cougar from the Black Hills, South Dakota. J Wildlife Manage.2005;69:818-820. 28. Stoner DC, Rieth WR, Wolfe ML, Mecham MB, Neville A. Long-distance dispersal of a female cougar in a basin and range landscape. J Wildlife Manage.2008;72:933-939. 29. Ricketts TH. The matrix matters: effective isolation in fragmented landscapes. Am Nat.2001;158:87-99. 30. Anderson Jr CR, Lindzey FG, McDonald DB. Genetic structure of cougar populations across the Wyoming Basin: metapopulation or megapopulation. J Mammal. 2004;85:1207-1214. 31. McRae BH, Beier P, Dewald LE, Huynh LY, Keim P. Habitat barriers limit gene flow and illuminate historical events in a wide-ranging carnivore, the American puma. Mol Ecol.2005;14:1965-1977. 32. Prevedello JA, Vieira MV. Does the type of matrix matter? A quantitative review of the evidence. Biodivers. Conserv.2010;19:1205-1223. 131 Texas Tech University, Anthony J. Giordano, May 2015 33. Watling J, Nowakowski AJ, Donnelly MA, Orrock JL. Meta-analysis reveals the importance of matrix composition for animals in fragmented habitat. Global Ecol Biogeogr.2011;20:209-217. 34. Silver S, Ostro LET, Marsh LK, Maffei L, Noss AJ, Kelly M. The use of camera traps for estimating jaguar (Panthera onca) abundance and density using capture/recapture analysis. Oryx2004;38:148-154. 35. Maffei L, Noss AJ, Silver SC, Kelly MJ. Abundance/ density case study: jaguars in the Americas. In: O’Connell AF, Nichols JD, Karanth KU. Camera traps in animal ecology. Tokyo, Japan: Springer Japan; 2011. pp. 119-144. 36. Caso A, Lopez-Gonazlez C, Payan E, Eizirik E, Oliveira TG, Leite-Pitman R, Kelly M & Valderrama C. Panthera onca. IUCN Red List of Threatened Species Version 2013.2. 2013. Available: http://www.iucnredlist.org/ 37. Sanderson EW, Redford KH, Chetkiewicz C-LB, Medellin RA, Rabinowitz AR, Robinson JG, Taber AB. Planning to save a species: the jaguar as a model. Conserv Biol.2002;16:58-72. 38. Culver M, Johnson WE, Pecon-Slattery J, O’Brien SJ. Genomic ancestry of the American puma (Puma concolor). J Hered.2000;91:186-197. 39. Eizirik E, Kim J-H, Menotti-Raymond M, Crawshaw PG, O’Brien SJ, Johnson WE. Phylogeography, population history, and conservation genetics of jaguars (Panthera onca, Mammalia, Felidae). Mol Ecol.2001;10:65-79. 40. Crawshaw PG. A personal view on the depredation of domestic animals by large cats in Brazil. Nat Conservacao.2003;1:71-73. 41. Paviolo A, De Angelo CD, Di Blanco YE, Di Bitetti MS. Jaguar Panthera onca population decline in the Upper Parana Atlantic Forest of Argentina and Brazil. Oryx 2008;42:554-561. 42. Lyra-Jorge MC, Ribeiro MC, Ciocheti G, Tambosi LR, Pivello VR. Influence of multi-scale landscape structure on the occurrence of carnivorous mammals in a human-modified savanna, Brazil. Eur J Wildlife Res.2009;56:359-368. 132 Texas Tech University, Anthony J. Giordano, May 2015 43. Haag T, Santos AS, Sana DA, Morato RG, Cullen Jr L, Crawshaw Jr PG, De Angelo C, Di Bitetti MS, Salzano FM, Eizirik E. The effect of habitat fragmentation on the genetic structure of a top predator: loss of diversity and high differentiation among remnant populations of Atlantic Forest jaguars. Mol Ecol. 2010;19:4906-4921. 44. De Angelo C, Paviolo A, Di Bitetti M. Differential impact of landscape transformation on pumas (Puma concolor) and jaguars (Panthera onca) in the Upper Parana Atlantic Forest. Divers Distrib2011;17:422-436. 45. Rabinowitz A, Zeller K. A range-wide model of landscape connectivity for the jaguar, Panthera onca. Biol Conserv.2010;143:939-945. 46. Swarts FA, editor. The Pantanal: understanding and preserving the world’s largest wetland. St. Paul, Minnesota: Paragon House Publishers; 2000 47. Harris MB, Tomas W, Mourao G, Da Silva CJ, Guimaraes E, Sonoda F, Fachim E.Safeguarding the Pantanal wetlands: threats and conservation initiatives. Conserv Biol. 2005;19:714-720. 48. Yanosky A. The challenge of conserving a natural Chaco habitat in the face of severe deforestation pressure and human development needs. In: Lambert P, Nickson A, editors. The Paraguay Reader: History, Culture, Politics (The Latin America Readers). Durham, NC: Duke University Press; 2012. pp. 376-382. 49. Caldas MM, Goodin D, Sherwood S, Campos Krauer JM, Wisely SM. Land-cover change in the Paraguayan Chaco: 2000-2011. J Land Use Sci. 2013, DOI: 10.1080/1747423X.2013.807314. 50. Zardini EM. Paraguay’s floristic inventory. Res Explor. 1993;9:128-13151. 51. Bucher EH, Huszar PC. Sustainable management of the Gran Chaco of South America: ecological promise and economic constraints. J Environ Manage. 1999;57:99-108. 52. Sanchez TF. The Climate of Paraguay. In: Gorham JR, editor. Paraguay Ecological Essays. Miami, Florida: Academy of the Arts and Sciences of the Americas; 1973. pp. 33-38. 133 Texas Tech University, Anthony J. Giordano, May 2015 53. Spichiger R, Ramella L. The forests of the Paraguayan Chaco. In: Holm-Nielsen LB, Nielsen IC, Balslev H. Tropical Forests: Botanical Dynamics, Speciation, and Diversity. London, UK: Academic Press; 1989. pp. 259-270. 54. Navarro G, Molina JA, de Molas LP. Classification of the forests of the northern Paraguayan Chaco. Phytocoenologia 2006;36:473-508. 55. Giordano AJ, et al. [Chapter 2]. Adaptation of noninvasive genetic techniques for identifying jaguars (Panthera onca) and pumas (Puma concolor) in the Gran Chaco: practical considerations and implications for sampling. 56. Haag T, Santos AS, De Angelo C, Srbek-Araujo AC, Sana DA, Morato RG, Salzana FM, Eizirik E. Development and testing of an optimized method for DNA-based identification of jaguar (Panthera onca) and puma (Puma concolor) faecal samples for use in ecological and genetic studies. Genetica2009;136:505-512. 57. Altschul, SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403-410. 58. Benson DA, Karsh-Mizrachi I, Lipman DJ, Ostell J, Wheeler DL. GenBank. Nucleic Acids Res. 2005;33:D34-D38. 59. Menotti-Raymond MM, David VA, Watcher LL, Butler JM, O’Brien SJ. A genetic linkage map of microsatellites in the domestic cat (Felis catus). Genomics 1999;57:9-23. 60. Valiere N. GIMLET: a computer program for analyzing genetic individual identification data. Mol Ecol Notes.2002;2:377-379. 61. Paetkau D, Waits LP, Clarkson PL, Craighead L, Vyse E, Ward R, Strobeck C. Variation in genetic diversity across the range of North American brown bears. Conserv Biol.1998;12:418-429. 62. Waits, LP, Luikart G, Taberlet P. Estimating the probability of identity among genotypes in natural populations: cautions and guidelines. Mol Ecol.2001;10:249-256. 134 Texas Tech University, Anthony J. Giordano, May 2015 63. Raymond M, Rousset F. GENEPOP (Version 1.2): Population genetics software for exact tests and ecumenicism. J Hered.1995;86:248-249. 64. Rousset F. Inferences from spatial population genetics. In: Balding DJ, Bishop M, Cannings C, editors. Handbook of statistical genetics. 3rd ed. Chichester, UK: Wiley Press; 2007. pp. 945-979. 65. Rousset F. GENEPOP’007: a complete re-implementation of the GENEPOP software for Windows and Linux. Mol Ecol Resour. 2008;8:103-106. 66. Goudet J. FSTAT (Version 1.2): A computer program to calculate F-statistics. J Hered. 1995;86:485-486. 67. Goudet, J. FSTAT: a program to estimate and test gene diversities and fixation indices. Lausanne, Switzerland: Institute of Ecology; 2002. 68. Louis EJ, Dempster ER. An exact test for Hardy-Weinberg Equilibrium and multiple alleles. Biometrics 1987;43:805-811. 69. Guo SW, Thompson EA. Performing the exact test of Hardy-Weinberg proportions for multiple alleles. Biometrics 1992;48:361-372. 70. Rice WR. Analyzing tables of statistical tests. Evolution 1989;43:223-225. 71. Peakall R, Smouse PE. GENALEX 6: genetic analysis in Excel. Population genetic software for teaching and research. Mol Ecol Notes.2006;6:288-295. 72. Petit RJ, Mousadik EL, Pons O. Identifying populations for conservation on the basis of genetic markers. Conserv Biol.1998;12:844-855. 73. Kalinowski ST. Counting alleles with rarefaction: private alleles and hierarchical sampling designs. Conserv Genet2004;5:539-543. 74. Kalinowski ST. HP-Rare: a computer program performing rarefaction on measures of allelic diversity. Mol Ecol Notes2005;5:187-189. 75. Nei M. F-statistics and analysis of gene diversity in subdivided populations. Ann Hum Genet. 1977;41:225-233. 76. Nei M. Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics1978;89:583-590. 135 Texas Tech University, Anthony J. Giordano, May 2015 77. Weir BS, Cockerham CC. Estimating F-statistics for the analysis of population structure. Evolution1984;38:1358-1370. 78. Slatkin M. A measure of population subdivision based on microsatellite allele frequencies. Genetics1995;139:457-462. 79. Michalakis Y, Excoffier L. A generic estimation of population subdivision using distances between alleles with special interest to microsatellite loci. Genetics1996;142:1061-1064. 80. Jost L. GST and its relatives do not measure differentiation. Mol Ecol.2008;17:4015-4026. 81. Verity R, Nichols RA. What is genetic differentiation, and how should we measure it – GST, D, neither, or both? Mol Ecol. 2014; doi: 10.1111/mec.12856. 82. Crawford NG.SMOGD: software for the measurement of genetic diversity. Mol Ecol Resour.2010;10:556-557. 83. Nei M, Chesser RK. Estimation of fixation indexes and gene diversities. Ann Hum Genet.1983;47:253-259. 84. Nei, M. Molecular Evolutionary Genetics. New York, NY: Columbia University Press; 1987. 85. Hedrick PW. A standardized genetic differentiation measure. Evolution2005;59:1633-1638. 86. Meirmans PG, Hedrick PW. Assessing population structure: FST and related measures. Mol Ecol Resour.2011;11:5-18. 87. Heller R, Siegismund HRS. Relationship between three measures of genetic differentiation: GST, DEST, and G’ST: how wrong have we been? Mol Ecol. 2009;18:2080-2083. 88. Meirmans PG. Using the AMOVA framework to estimate a standardized genetic differentiation measure. Evolution2006;60:2399-2402. 89. Wright S. Isolation by distance. Genetics 1943;28:114-138. 90. Rousset F. Genetic differentiation and estimation of gene flow from F-statistics under isolation by distance. Genetics 1997;45:1219-1228. 136 Texas Tech University, Anthony J. Giordano, May 2015 91. Mantel N. The detection of disease clustering and a generalized linear regression approach. Cancer Res. 1967;27:209-220. 92. Smouse PE, Long JC, Sokal RR. Multiple regression and correlation extensions of the Mantel test of matrix correspondence. Syst Zool. 1986;35:627-632. 93. Nei M. Genetic distance between populations. Am Nat. 1972;106:283-392. 94. Smouse PE, Peakall R. Spatial autocorrelation analysis of individual multiallele and multilocus genetic structure. Heredity1999;82:561-573. 95. Pritchard JK, Stephens M, Donnelly P. 2000. Inference of population structure using multilocus genotype data. Genetics 155;945-959. 96. Pritchard JK, Wen X, Falush D. Documentation for structure software: Version 2.3. 2010. Available: http://pritch.bsd.uchicago.edu/structure.html. 97. Waples RS, Gaggiotti O. What is a population? An empirical evaluation of some genetic methods for identifying the number of gene pools and their degree of connectivity. Mol Ecol. 2006;15:1419-1439. 98. Evanno G, Regnaut S, Goudet J. Detecting the number of clusters of individuals using the software STRUCTURE : a simulation study. Mol Ecol. 2005;14:2611-2620. 99. Jakobsson M, Rosenberg NA. CLUMPP: a cluster matching and permuation program for dealing with label switching and multimodality in analysis of population structure. Bioinformatics2007;23:1801-1806. 100. Peakall R, Smouse PE. GenAlEx 6.5: genetic analysis in Excel. Ppulation genetic software for teaching and research- an update. Bioinformatics2012;28:25372539. 101. Di Marco M, Boitani L, Mallon D, Hoffmann M, Lacucci A, Meijaard E, Visconti P, Schipper J, Rondinini C. A retrospective evaluation of the global decline of carnivores and ungulates. Conserv Biol.2014;28:1109-1118. 102. Weber W, Rabinowitz A. A global perspective on large carnivore conservation. Conservation Biology 1996;10:1046-1055. 137 Texas Tech University, Anthony J. Giordano, May 2015 103. Carroll C, Noss RF, Paquet PC. Carnivores as focal species for conservation planning in the Rocky Mountain region. Ecol Appl. 2001;11:961-980. 104. Wikramanyake E, McKnight M, Dinerstein E, Joshi A, Gurung B, Smith D. Designing a conservation landscape for tigers in human-dominated environments. Conserv Biol. 2004;18:839-844. 105. Peacock E, Derocher AE, Thiemann GW, Stirling I. Conservation and management of Canada’s polar bears (Ursus maritimus) in a changing Arctic. Can J Zool. 2011;89:371-385. 106. Ruiz-Garcia M, Payan E, Murillo A, Alvarez D. DNA microsatellite characterization of the jaguar (Panthera onca) in Colombia. Genes Genet Syst.2006;81:115-127. 107. Miotto RA, Cervini M, Figueiredo MG, Begotti RA, Galetti Jr PM. Genetic diversity and population structure of pumas (Puma concolor) in southeastern Brazil: implications for conservation in a human-dominated landscape. Conserv Genet. 2011;12:1447-1455. 108. Frankham R, Ballou JD, Briscoe DA. Introduction to conservation genetics. 2nd ed. Cambridge, UK: Cambridge University Press; 2010. 109. Hartl DL, Clark AG. Principles of Population Genetics. Sunderland, MA: Sinauer Associates; 2007. 110. Gaggiotti OE, Lange O, Rassmann K, Gliddon C. A comparison of two indirect methods for estimating average levels of gene flow using microsatellite data. Mol Ecol. 1999;8:1513-1520. 111. Whitlock MC. G’ST and D do not replace FST. Mol Ecol.2011;20:1083-1091. 112. Soisalo M, Cavalcanti S. Estimating the density of a jaguar population in the Brazilian Pantanal using camera-traps and capture-recapture sampling in combination with GPS radio-telemetry. Biol Conserv. 2006;129:487-496. 113. Wright S. Evolution in Mendelian populations. Genetics 1931;16:97-159113. Franklin JR. Evolutionary change in small populations. In: Soule ME, Wilcox BA, editors. Sunderland, MA: Sinauer Associations; 1980. pp. 135-149. 138 Texas Tech University, Anthony J. Giordano, May 2015 114. Mace GM, Lande R. Assessing extinction threats: toward a reevaluation of the IUCN threatened species categories. Conserv Biol. 1991;5:148-157. 115. Mills LS, Allendorf FW. The one-migrant-per-generation rule in conservation and management. Conserv Biol. 1996;10:1509-1518. 116. Vucetich JA, Waite TA. Is one migrant per generation sufficient for the management of fluctuating populations? Anim Conserv. 2000;3:261-266. 117. Carron JM. The Pantanal of Paraguay. In: Swarts FA, editor. The Pantanal: understanding and preserving the world’s largest wetland. St. Paul, Minnesota: Paragon House Publishers; 2000. pp. 55-67. 118. Killeen TJ, Guerra A, Calzada M, Correa L, Calderon V, Soria L, Quezada B, Steininger MK. Total historical land-use change in eastern Bolivia: who, where, when, and how much? Ecol Soc2008;13:36. http://www.ecologyandsociety.org/vol13/iss1/art36/. 119. Crawshaw PG Jr. Comparative ecology of ocelot (Felis pardalis) and jaguar (Panthera onca) in a protected subtropical forest in Brazil and Argentina. Ph.D. Dissertation, University of Florida. 1995. 120. ZimmermanA. Cattle ranchers’ attitudes to conflicts with jaguars Panthera onca in the Pantanal of Brazil. Oryx 2005;39:406-412. 121. Giordano AJ. Estado y conservación del jaguar (Panthera onca) en el Chaco Paraguayo. In: Rumiz DI, Polisar J, Maffei L, editors. El futuro del jaguar en el Gran Chaco – Situacion en Bolivia, Paraguay, and Argentina. Santa Cruz, Bolivia: Wildlife Conservation Society; 2011. pp 17-18. 122. Cavalcanti SMC, De Azevedo FCC, Tomas WM, Boulhosa RLP, Crawshaw Jr PG. The status of the jaguar in the Pantanal. Cat News, Special Issue 2012;7:29-34. 123. Giordano AJ, Cameroni NM, Ramirez F, Nielsen CK. Jaguar records from the south-central Paraguayan Chaco. Cat News 2014;60:38-40. 139 Texas Tech University, Anthony J. Giordano, May 2015 Figure 4.1. Map depicting the relative location of core sampling areas. The red polygon depicts where sampling occurred in Gran Chaco Jaguar Conservation Unit (left, Paraguay), while the green polygon depicts where samples were collected in and the Pantanal Jaguar Conservation Unit (right, Brazil). The closest samples from these areas were approximately 280 km apart. 140 Texas Tech University, Anthony J. Giordano, May 2015 141 Texas Tech University, Anthony J. Giordano, May 2015 Table 4.1. Measures of genetic diversity and for 8 microsatellite loci for 40 individual jaguar scat samples from the Paraguayan dry Chaco (N = sample size, SR = allele size range (base pairs), AN = observed number of alleles, HO = Observed Heterozygosity, HE = Expected Heterozygosity, uHE = unbiased I = Shannon’s Information Content, PA = Private Alleles, and P(ID-SIB) = probability of identity for siblings) Locus N SR (bp) AN HO HE uHE I P(ID)SIB FCA77 40 162-182 8 0.750 0.829 0.839 1.856 0.3528 FCA126 28 166-182 5 0.714 0.742 0.755 1.457 0.3941 FCA161 40 184-220 12 0.919 0.757 0.768 1.855 0.3680 FCA211 40 135-145 6 0.850 0.726 0.735 1.459 0.4206 FCA229 39 186-206 10 0.692 0.643 0.651 1.481 0.4607 FCA441 33 162-182 9 0.606 0.791 0.803 1.770 0.3738 FCA453 38 187-231 12 0.632 0.662 0.671 1.623 0.3899 FCA678 37 243-259 7 0.622 0.718 0.728 1.468 0.4353 142 Texas Tech University, Anthony J. Giordano, May 2015 Table 4.2. Comparisons of overall measures of genetic diversity for individual jaguars originating from the Paraguayan Chaco and Brazilian Pantanal based on scat collections (AN = mean observed number of alleles per locus, AR = estimate of allelic richness, HO = observed heterozygosity, HE = expected heterozygosity, I = Shannon’s information content, PAO = observed number of private Alleles, PAE = estimated number of private alleles, and P(ID-SIB) = probability of identity across all loci) Population AN AR * HO HE I PAO PAE* P(ID)SIB Chaco 8.625 4.08 0.723 0.733 1.621 4.375 2.06 6.29 x 10-4 Pantanal 6.000 4.77 0.523 0.780 1.638 1.750 2.75 5.25 x 10-4 *Estimates accounting for differences in sample size 143 Texas Tech University, Anthony J. Giordano, May 2015 Table 4.3. Pairwise estimates of measures of population differentiation between jaguars in the Paraguayan Chaco (PC) and the Brazilian Pantanal (BP) [FST, GST, G’ST, Nei’s G’ST, & DEST & G’’ST] and the effective number of migrants (Nm) for each locus and the mean across all loci. FST GST Nei’s G’ST G’ST(H) G’’ST DEST Nm FCA77 0.053 0.029* 0.056* 0.307* 0.327* 0.287* 4.487 FCA126 0.043 0.018 0.036 0.213~ 0.228 0.199~ 5.532 FCA161 0.069 0.047** 0.089** 0.486** 0.509** 0.461** 3.386 FCA211 0.064 0.041** 0.078** 0.356** 0.381** 0.328** 3.634 FCA229 0.091 0.042* 0.081* 0.255** 0.285** 0.222** 2.510 FCA441 0.092 0.055* 0.104* 0.573** 0.595** 0.548** 2.455 FCA453 0.060 0.030* 0.059* 0.261* 0.283* 0.238* 3.951 FCA678 0.094 0.065** 0.122** 0.587*** 0.612*** 0.558*** 2.399 Total/ Mean 0.071*** 0.041*** 0.079*** 0.374*** 0.399*** 0.348*** 3.284 Locus * Significant at α = 0.05; **Significant at α = 0.01; ***Significant at α = 0.001; ~ = almost significant at α = 0.05 (i.e, p~0.05) 144 Texas Tech University, Anthony J. Giordano, May 2015 Figure 4.2a. Mantel test of the linear geographical distance (x) and Nei’s genetic distance (y) for 50 individual jaguars originating from the Paraguayan Chaco and Brazilian Pantanal (p<0.001). 145 Texas Tech University, Anthony J. Giordano, May 2015 146 Texas Tech University, Anthony J. Giordano, May 2015 Figure 4.2b. Mantel test of the log-transformed geographical distance (x) and Nei’s genetic distance (y) for 50 individual jaguars originating from the Paraguayan Chaco and Brazilian Pantanal (p<0.001). 147 Texas Tech University, Anthony J. Giordano, May 2015 148 Texas Tech University, Anthony J. Giordano, May 2015 Figure 4.3. Regression coefficient estimates of geographical vs. genetic distance plotted over multiple expanding distance classes (km) of pairwise comparisons between jaguar samples. The graph demonstrates weak (r stabilizes at ~0.016) but significant spatial autocorrelation between genetic and geographical distance 149 Texas Tech University, Anthony J. Giordano, May 2015 150 Texas Tech University, Anthony J. Giordano, May 2015 Figure 4.4. Mean population probability assignment (q) for 50 individual jaguars for 10 iterations of K = 2 where population origin was specified. The dark orange coloration represents the probability that an individual is assigned to the population originating from the Gran Chaco of Paraguay, while the green represents the probability an individual is assigned to the Brazilian Pantanal. The 10 individual jaguars originating from the Pantanal are all toward the right of the graph. 151 Texas Tech University, Anthony J. Giordano, May 2015 152 Texas Tech University, Anthony J. Giordano, May 2015 Figure 4.5. Plot of variation in ΔK, or the mean of the absolute value of the 2nd order of the likelihood distribution (|Mean(L”(K)|) over the standard deviation of the mean likelihood values (SD (L(K)), with successive values for K (K = 2-9); ΔK is maximized for K=2. 153 Texas Tech University, Anthony J. Giordano, May 2015 154 Texas Tech University, Anthony J. Giordano, May 2015 Figure 4.6. Mean population probability assignment (q) for 50 individual jaguars for 10 iterations of K = 2 where population origin wasn’t specified a priori. The dark orange coloration represents the probability that an individual is assigned to the population originating from the Gran Chaco of Paraguay, while the green represents the probability an individual is assigned to the Brazilian Pantanal. The 10 individual jaguars originating from the Pantanal are all toward the right of the graph. 155 Texas Tech University, Anthony J. Giordano, May 2015 156 Texas Tech University, Anthony J. Giordano, May 2015 Figure 4.7a, b. Maps of varying extent depicting the geographical location of jaguar scat samples collected from the Paraguayan Chaco (yellow pins, Alto Paraguay) and Brazilian Pantanal (green and red pins, Mato Grosso do Sul). Both maps show in detail the extensive deforestation and development that has occurred in both the Chaco uplands and Pantanal floodplains. 157 Texas Tech University, Anthony J. Giordano, May 2015 158 Texas Tech University, Anthony J. Giordano, May 2015 159 Texas Tech University, Anthony J. Giordano, May 2015 160 Texas Tech University, Anthony J. Giordano, May 2015 161 Texas Tech University, Anthony J. Giordano, May 2015 CHAPTER VI SUMMARY This research project represents the first investigation into the conservation status, ecology, and population genetics of the jaguar in the country of Paraguay. Moreover, at its core it is one of only a few detailed scientific investigations of the jaguar in the Gran Chaco. First, we synthesized all known direct and indirect distributional records of the jaguar for the country of Paraguay. We included records based on secondary interviews, indirect sign, and observations made by eyewitnesses and ourselves. We then mapped the distribution of jaguars across Paraguay’s major ecoregions and discussed the threats jaguars face across the country, with an emphasis on the Gran Chaco. In this latter region, unsustainable cattle production driven largely by high beef prices, coupled with land prices relatively cheaper than in adjacent countries, represent the ultimate threats to jaguars, forests, and jaguar prey alike. In eastern Paraguay, existing agricultural production for soybeans and other crops, habitat fragmentation and isolation, conflict over land rights, illegal grazing and hunting, lack of resources for protected areas, lack of regional reforestation efforts, and inadequate enforcement of environmental laws, are among the biggest existing jaguars and the recolonization of jaguars to new regions. We recommend a broad multi-faceted approach to threat mitigation. This might include increasing public awareness and engagement, programs to implement conflict-mitigation and prevention measures, greater enforcement of deforestation and jaguar persecution laws, elimination of loopholes that permit continued deforestation in areas, a long-term jaguar monitoring program, greater resources and infrastructure for protected areas, and more economic incentives to explore alternative and additional land uses that encourage the protection of more habitat, including the production and marketing of sustainable beef, are among the most important measures that could be taken to reduce threats to jaguar populations. Secondly, we collected several hundred large felid scat samples (n=554) in the northern dry Paraguayan Chaco to test the efficacy of noninvasive genetic sampling 162 Texas Tech University, Anthony J. Giordano, May 2015 for monitoring jaguars and pumas in the region. We used a primer set optimized for identifying and differentiating jaguars and pumas based on the ATP-6 region of the mtDNA genome. We assessed potential field and laboratory factors that might influence our ability to identify the origin of scats, and evaluated species and demographic patterns of our samples in two distance categories with respect to a major protected area in the Chaco. We unambiguously identified 95% of our samples as to species origin; 54% were jaguar, 34% were puma, and the remaining samples yielding results were of canid or small cat origin. We found no evidence that initial dry volume or long-term storage on dry samples had any effects on success, but that humanintroduced variability and/or the time interval between DNA extraction and PCR amplification, may be important. We identified the sex of 77% of all large felid samples, noting a near 1:1 ratio of male to female pumas, but a highly skewed 5:1 ratio of male to female jaguars. We also identified significantly more puma samples than jaguar samples at distances of 5 km or more from a protected area, nearly all of the latter of which were males. We are unclear if demographic sampling biases may exist using noninvasive genetic sampling or if our findings reflect the population’s composition; however, we believe use of this methodology to be an effective means of surveying large felids in the Gran Chaco. Third, we applied these species identification techniques to a noninvasive capture-recapture sampling framework in order to estimate the abundance of jaguars at Defensores del Chaco National Park. This was the first such effort for the country of Paraguay, and the first noninvasive genetic sampling investigation of jaguar abundance in the Gran Chaco. We collected 205 jaguar scats across three independent sampling events and tested the efficacy of eight microsatellites to facilitate the individual identification of jaguars among the samples collected. We found that seven worked well enough to permit the identification of 12-19 individual genotypes for each sampling event. We used capwire, a likelihood-based CR estimator that is little biased by small population sizes and low sampling effort, and performs better when capture heterogeneity is present. After applying buffers based on the home range movements of jaguars from the region to create realistic effective sampling areas, we 163 Texas Tech University, Anthony J. Giordano, May 2015 calculated a density estimate of D = 0.72 jaguars/ 100 km2, or one jaguar per 137 km2 This led to population size of N = 58 jaguars for Chaco Defensores National Park. We discussed the possible implications of effective sampling area, type of sampling technique, and methodological assumptions in accounting for differences between estimates of jaguar density in the Bolivian and Paraguayan Chaco despite the proximity of the regions, but concluded that our estimates of jaguar density are among the lower estimates of density for a free-ranging jaguar population across the distribution of the species. Finally, we examined in detail the genetic of jaguars in the Paraguayan Dry Chaco and Brazilian Pantanal. We also conducted the first transboundary investigation of genetic connectivity between jaguar populations in these two regions. Using the same eight microsatellite markers, we found high levels of heterozygosity and allelic diversity for jaguars in both the Chaco and Pantanal, supporting prior findings that the genetic diversity of jaguars across their range is relatively high. We found evidence of genetic connectivity between jaguars in the Paraguayan Chaco and the Brazilian Pantanal. Bayesian analyses in STRUCTURE lacking population specificity suggested jaguars in these two areas constitute a single panmictic population. Although we found moderate evidence of isolation-by-distance or clinal effects, using additional measurement of differentiation, we also found evidence of some population structure between jaguars in these two relatively close regions. This is not inconsistent with the more recent emergence of population structure between these areas, possibly due to the very high levels of deforestation and agricultural development that now separate these two areas. Future intensification of development and loss of forest across this region could lead to increased isolation between jaguars in these two regions and a decline in jaguar gene flow. 164 Texas Tech University, Anthony J. Giordano, May 2015 APPENDIX A. JAGUAR RECORDS FROM PARAGUAY Location (No. Records) Ecoregion Dept Type Date Bahia Negra: KM 34, Linea 1 DC ALP S 2002 Estancia 11 de Junio HC PRH S 2006 Estancia Alegria AF SPE O 2002 Estancia Aurora* HC PRH O 2011 Estancia Cambushi HC NEE I 2004 Estancia Campo Grande, Linea 28 DC ALP S 2002 Estancia Chovereca, Linea 28 DC ALP S 2002 Estancia Don Luis AF SPE O 2002 Estancia El Ciebo (Reserva Natural)* DC ALP O 2010 Estancia El Ciervo DC ALP O 2005 Estancia Faro Moro* DC BOQ O 2007-2010 Estancia Fortin Patria (Reserva Naural)* PA ALP O 2007-2010 Estancia Garay CE CON O 2006 Estancia La Gama DC BOQ I 2004 Estancia La Manada HC PRH O 2006 Estancia La Petrona HC PRH C 2009 Estancia Menno (Campo Maria Cuenca de Yacare Sur)* Estancia Paso Curuzo HC PRH S AF SPE S 1998, 2000-2001 2005 Estancia Quinto Potrero AF CAA O 2002 Estancia Sorpressa* DC ALP C 2002,2009 165 Texas Tech University, Anthony J. Giordano, May 2015 Estancia Yatamasit HC PRH I 2001 Estancia Santo Domindo/ Santa Ana/ Cero Cora Estancia Uruguaya DC ALP O 2004 DC ALP S 2002 “Fte. Olimpo” PA ALP O 2005 Kamba Aka (Chovereca Region, Linea 14) DC ALP I 2002 Laguna Pora (Chovereca Region, Linea 2) DC ALP O 2009 Parque Nacional Cerro Cabrera/ Timane* DC ALP S 2007,2008 Parque Nacional Cerro Chovereca DC ALP I 2008 Parque Nacional Chaco Defensores* DC ALP O 2007-2012 Parque Nacional Laguna Ganzzo (Proposed; S.A. Victoria) Parque Nacional Laguna Inmakata (Proposed) Parque Nacional Medanos del Chaco* DC PRH S 2001 DC ALP I 2001 DC BOQ C 2007,2011 Parque Nacional Paso Bravo CE CON C 2009 Parque Nacional Rio Negro* DC ALP C 2009 Parque Nacional San Luis CE CON O 1996 Parque Nacional Teniente A. Enciso* DC BOQ O 2006,2008 Parque Nacional Tifunque* DC PRH O 2003 “Rancho K” DC ALP I 2000 Refugio Biologico Itakyry (ITAIPU) AF APA S 2003 Refugio Biologico Carapa (ITAIPU) AF CAN S 2004 Refugio Biologico Itabo (ITAIPU) AF APA O 2009 166 Texas Tech University, Anthony J. Giordano, May 2015 Refugio Biologico Limoy (ITAIPU) AF APA S 2004 Reserva Ecologia Riacho Yacare [S.A. Victoria] Reserva Natural Los Tres Gigantes* HC PRH O 2007 PA ALP O 2008-2011 Reserva Indigena Nu Guazu DC ALP C 2007 Reserva Natural Arroyo Blanco AF AMA O 2000 Reserva Natural Bosque Mbaracayu* AF/CE CAN O 2005-2011 Reserva Natural Campo Iris DC BOQ I 2008 Reserva Natural Canada del Carmen DC BOQ S 2003 Reserva Natural Cerrados del Tagatiya CE CON I 2004 Reserva Natural Estrella* HC/CE CON O 2007-2010 Reserva Natural Fortin Salazar HC PRH I 2009 Reserva Natural Golondrina II* AF CAA O 2000 Reserva Natural ITABO* AF APA I Reserva Natural Jaguarete Pora* DC ALP O 20012002, 2009 2009 Reserva Natural Ka’i Rague* AF APA O, I Reserva Natural Lote 1 (Corredor de des del Chaco) Reserva Natural Morombi* DC ALP S 2006, 2011 2008-2011 AF CAA O 2007-2012 Reserva Natural Riacho Florida 2* DC ALP O 2008 Reserva Natural Tabucai* AF APA C 2006 Reserva Natural Tagitiya Mi* CE CON C 2003,2007 Reserva Natural Toro Mocho* DC BOQ O 2012 167 Texas Tech University, Anthony J. Giordano, May 2015 Reserva Natural Ypeti (Golondrina III?) AF CAA C 2011 Reserva Natural Estero Milagros* HC SPE O 2003 Victoria S.A.* HC PRH O 2010-2011 Yaguarete Forests AF SPE I 2000 Ecoregion:: HC = Humid Chaco, AF = Upper Parana Atlantic Forest, CE = Cerrado, PA = Pantanal; DC = Dry Chaco; Department:: NEE = Neembucu, CAA = Caazapa, PRH = Presidente Hayes, SPE = San Pedro, ALP = Alto Paraguay, APA = Alto Parana, CON = Concepcion, CAN = Canindeyu, AMA = Amambay, BOQ = Boqueron; Type (of Evidence):: O = observation, I = secondhand inteview, S = sign, C = combined sign/ secondhand interview; * = multiple records from the same location 168 Texas Tech University, Anthony J. Giordano, May 2015 APPENDIX B. SAMPLE RECORDS USED IN POPULATION GENETICS ANALYSIS Sample No. Region Latitude Longitude Collection Date PY120 PARAGUAY -60.693337 -20.303322 November 2008 PY129 PARAGUAY -60.577681 -20.356786 November 2008 PY014b PARAGUAY -60.933769 -20.175573 August 2008 PY152 PARAGUAY -59.758996 -20.289594 April 2009 PY165 PARAGUAY -59.758745 -20.174466 April 2009 PY167 PARAGUAY -59.758529 -20.053428 April 2009 PY169 PARAGUAY -60.138227 -19.983528 April 2009 PY175 PARAGUAY -59.974311 -20.619714 April 2009 PY178 PARAGUAY -59.992479 -20.614587 April 2009 PY182 PARAGUAY -60.258386 -20.504336 April 2009 PY191 PARAGUAY -60.534362 -20.329669 April 2009 PY206 PARAGUAY -60.872523 -20.217075 April 2009 PY262 PARAGUAY -60.107388 -19.983585 June 2009 PY027 PARAGUAY -60.718864 -20.142720 August 2008 PY280 PARAGUAY -60.982306 -20.142720 June 2009 PY284 PARAGUAY -60.939147 -20.171982 June 2009 PY306 PARAGUAY -60.624969 -20.334965 June 2009 PY328 PARAGUAY -60.533907 -20.298640 June 2009 PY329 PARAGUAY -60.532840 -20.218448 June 2009 169 Texas Tech University, Anthony J. Giordano, May 2015 PY367 PARAGUAY -57.269118 -22.571228 August 2009 PY376 PARAGUAY -58.970260 -21.690968 August 2009 PY386 PARAGUAY -60.533286 -20.255381 August 2009 PY389 PARAGUAY -60.532707 -20.211278 August 2009 PY418 PARAGUAY -60.595568 -19.736780 August 2009 PY426 PARAGUAY -60.131510 -20.563503 August 2009 PY428 PARAGUAY -60.158276 -20.550990 August 2009 PY437 PARAGUAY -60.314481 -20.478258 August 2009 PY451 PARAGUAY -60.626625 -20.334243 August 2009 PY455 PARAGUAY -60.853205 -20.230196 August 2009 PY468 PARAGUAY -60.702951 -20.011575 August 2009 PY473 PARAGUAY -60.652070 -20.057817 August 2009 PY477 PARAGUAY -60.622665 -20.073114 August 2009 PY480 PARAGUAY -60.559742 -20.107701 August 2009 PY489 PARAGUAY -59.579442 -20.212054 August 2009 PY495b PARAGUAY -60.152341 -19.983546 August 2009 PY498 PARAGUAY -59.154324 -20.576760 August 2009 PY517 PARAGUAY -60.532353 -20.196575 August 2009 PY06a PARAGUAY -61.715937 -20.082071 July 2008 PY78 PARAGUAY -60.986165 -20.140114 August 2008 PY98 PARAGUAY -60.168839 -19.983515 November 2008 170 Texas Tech University, Anthony J. Giordano, May 2015 BR11 BRAZIL -56.371450 -19.751180 November 2008 BR18 BRAZIL -56.318330 -19.329310 November 2007 BR21 BRAZIL -56.345560 -19.747350 November 2007 BR37 BRAZIL -56.262460 -19.887860 February 2008 BR76 BRAZIL -56.297180 -19.947870 November 2008 BR77 BRAZIL -56.316760 -19.928070 November 2008 BR78 BRAZIL -56.321610 -19.876130 November 2008 BR84 BRAZIL -56.237610 -19.954210 November 2008 BR85 BRAZIL -56.871380 -19.770250 February 2008 BR86 BRAZIL -56.350660 -19.747030 February 2008 171