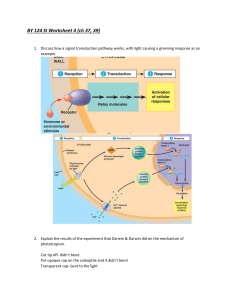
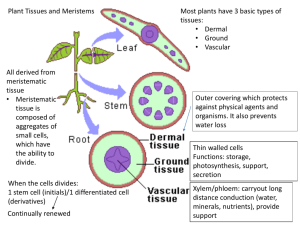
View/Open - Sacramento
advertisement

OVEREXPRESSION OF THE TOMATO PLASMA MEMBRANE H+-ATPASE, LHA2, IN ARABIDOPSIS THALIANA TO TEST ITS ROLE IN THE CONTROL OF PLANT GROWTH AND DEVELOPMENT A Thesis Presented to the faculty of the Department of Biological Sciences California State University, Sacramento Submitted in partial satisfaction of the requirements for the degree of MASTER OF SCIENCE in BIOLOGICAL SCIENCES (Molecular and Cellular Biology) by Robert Gar Bo Boyce FALL 2012 OVEREXPRESSION OF THE TOMATO PLASMA MEMBRANE H+-ATPASE, LHA2, IN ARABIDOPSIS THALIANA TO TEST ITS ROLE IN THE CONTROL OF PLANT GROWTH AND DEVELOPMENT A Thesis by Robert Gar Bo Boyce Approved by: __________________________________, Committee Chair Nicholas Ewing, Ph. D. __________________________________, Second Reader Thomas Peavy, Ph. D. __________________________________, Third Reader Hao Nguyen, Ph. D. ____________________________ Date ii Student: Robert Gar Bo Boyce I certify that this student has met the requirements for format contained in the University format manual, and that this thesis is suitable for shelving in the Library and credit is to be awarded for the thesis. __________________________, Graduate Coordinator Jamie Kneitel, Ph. D. Department of Biological Sciences iii ___________________ Date Abstract of OVEREXPRESSION OF THE TOMATO PLASMA MEMBRANE H+-ATPASE, LHA2, IN ARABIDOPSIS THALIANA TO TEST ITS ROLE IN THE CONTROL OF PLANT GROWTH AND DEVELOPMENT by Robert Gar Bo Boyce Plant plasma membrane H+-ATPases, such as LHA2, are primary active transporters that play a role in many physiological processes including maintenance of intra- and extracellular pH and cellular expansion. In order to gain a better understanding of plant growth and development this study focused on plant primary and lateral roots specifically looking at two areas of growth that the plasma membrane H+-ATPases effect, the Acid Growth Theory and lateral root initiation. The Acid Growth Theory suggests that the activation of plasma membrane H+-ATPases by auxin causes acidification of the apoplast, which causes loosening of the cell wall allowing turgor driven cell expansion. The other issue dealt with was lateral root initiation; currently there is no definitive early signal that triggers lateral roots. This study supports the hypothesis that activation of the H+-ATPase would cause both cell expansion according to the Acid Growth Theory and would be an early signal in lateral root initiation. To observe adequately the plasma membrane H+-ATPases, the development of two additional constitutively active iv overexpression lines were studied with two existing overexpression lines in Arabidopsis thaliana. These plant lines insured permanent activation by means of artificial truncation of the autoinhibitory domain and overexpression by coupling the LHA2 gene with the 32S promoter of the cauliflower mosaic virus. Results indicated that there was expression of the LHA2 transgene in Arabidopsis confirmed by RT-qPCR and that expression of the construct produced a fully functional H+ pump protein verified by fluorescent extracellular acidification activity assays using the pH sensitive fluorescent dye, dextran Oregon Green. The extracellular pH assay revealed that all overexpression lines had significantly lower extracellular pH (p<0.05), LHA2-2C at pH 4.73, LHA2-3C at pH 4.61, LHA2-6H at pH 4.44 and LHA2-12K at pH 4.43, compared to the wild-type control at pH 5.01. Phenotypic root results showed significant increases in primary root growth of all single copy homozygous overexpression lines (p<0.01), LHA2-2C, LHA2-3C, LHA2-6H and LHA2-12K with mean growth of 46.6 mm, 49.0 mm, 45.5 mm and 42.3 mm respectively compared to wild-type root growth at 39.2 mm in the 8 day primary root growth experiment. The 8 day lateral root density experiment showed increased densities of lateral roots in 3 of the 4 single copy homozygous overexpression lines (p<0.01). Mean root numbers of LHA2-2C at 1.39 lateral roots/cm (LR/cm), LHA2-3C at 1.49 LR/cm and LHA2-6H with 1.30 LR/cm, when compared to wild-type root at 1.16 LR/cm grown on M/S agar plates in our root growth assays. The wild-type and overexpression lines were also grown with the auxin transport regulators, TIBA and NPA in order to separate the effects of polar auxin flow and endogenous H+ pump activity. These results v showed that in the absence of polar auxin flow and endogenous pump activity, effects of the transgenic H+ pumps in the overexpression lines were able to retain growth in primary root length for 3 of the 4 overexpression lines, LHA2-3C, LHA2-6H and LHA2-12K. They also increased retention of lateral root densities in all of the overexpression lines when growing the plant lines on the auxin transport inhibitor, NPA and normalized to the DMSO vehicle control data. Results for plants grown on TIBA and normalized to the DMSO vehicle control, indicated that there were greater retention in lateral root density of the overexpression lines compared to the wild-type but decreased primary root retention which was contrary to our original hypothesis. To date, this study provides some of the most direct evidence that the function of the H+ pump is directly involved with acidification of the apoplast and cell expansion as stated by the Acid Growth Theory. This study also strengthens the hypothesis that the activity of the H+ pump by auxin activation creates transitory cytoplasmic alkalinization events, which may be required as one of the earliest signals to trigger the initiation of lateral roots in plants. _______________________, Committee Chair Nicholas Ewing, Ph. D. _______________________ Date vi ACKNOWLEDGEMENTS This thesis would not be possible without the help of several individuals who have contributed in both preparation and completion of this study. First I would like to express my gratitude for Dr. Nicholas Ewing whose knowledge, guidance and patience helped me to complete this research and thesis. I would also like to acknowledge and thank my committee, Dr. Thomas Peavy and Dr. Hao Nguyen for all their help on the thesis. I want to thank my friends and colleagues, Akihiro Tsuyada and Milo Careaga who initially trained me in my pursuit of research and for providing insight and knowledge. I would also like to acknowledge the support of Albert Deslisle and the scholarship and grants I had received from his foundation to fund this research. A special thanks to Dr. Maria Iturbide for her help with statistical analysis. Lastly, I would like to thank my family and my wife, Sherry, who have encouraged me through this entire process. vii TABLE OF CONTENTS Page Acknowledgements ................................................................................................................ vii List of Tables ........................................................................................................................... ix List of Figures ........................................................................................................................... x INTRODUCTION .................................................................................................................... 1 MATERIALS AND METHODS ............................................................................................ 20 RESULTS ............................................................................................................................... 31 DISCUSSION ......................................................................................................................... 82 Literature Cited ..................................................................................................................... 104 viii LIST OF TABLES Tables Page 1. Primer sequence data for use in PCR, RT-PCR and qPCR ……………………26 2. Segregation analysis for the single insertion lines LHA2-6 and LHA2-12 ..…. 37 3. Chi square (Χ2) test for LHA2-6 and LHA2-12 transgenic lines .......................38 4. Pfaffl Method for fold expression ………….………………………………….47 5. Summary of the pH analysis between the overexpression lines and the wild-type ............................................................................................................55 6. Lateral and primary root analyses of plants grown on MS agar plates at 8 dpg.65 7. Two-way ANOVA on primary root length .…………………………………. .76 8. Post Hoc Bonferroni analysis of the genotype for the primary roots.......... ………….77 9. Two-way ANOVA on lateral roots ……….…………………………………. .79 10. Post Hoc Bonferroni analysis of the genotype for the lateral roots ..................... …….80 ix LIST OF FIGURES Figures Page 1. LHA2/p2KGW7 Construct map …………… .………………………………. 21 2. A black walled fluorescent plate loaded with plants before obtaining pH fluorescent readings on a Promega Glomax plate reader ……………………. 30 3. Selection on 40 µg/ml kanamycin sulfate (14 days post-germination) .. ……. 32 4. Mendelian inheritance in the event of a single transgene insertion …………. 34 5. Demonstrates the expected outcome of the self-cross of a line containing two copies of the transgene ................................................ ………………………. 35 6. PCR detection of the LHA2 transgene in the transgenic overexpression lines and the wild-type parental line…………….…………………………………. 40 7. Reverse Transcriptase PCR from total RNA from transgenic and wild type Arabidopsis plants ………………………….……… .......... ………………… 42 8. A melt peak chart run after the gradient temperature analysis ………………. 45 9. The Pfaffl Method of Analysis was used to determine the fold expression of a target mRNA to that of a target gene .................................. ..…………… 46 10. Amplification chart showing the primer efficiency of the GAPDH primer set…………………. ................................................................................................ 48 11. Amplification chart of the primer efficiency for LHA2161 primer set ……… 49 12. Amplification chart of the primer efficiency for TIP41 primer set . ………… 50 13. Analysis of LHA2 mRNA expression using the Pfaffl Method ....................... 51 14. Analysis of LHA2 mRNA expression using the Pffafl method……………… 52 x 15. The standard curves of pH at different concentrations of dextran Oregon Green ....................................................................... …………………………. 54 16. Extracellular pH extrapolated from a 30 µm Oregon Green standard curve. ... 56 17. Transgenic and wild-type plants grown vertically in a growth chamber...… ... 61 18. Growth of transgenic and wild-type lines on MS agar plates ..................... …. 62 19. Total primary root length at 8 dpg. ......................... …………………………. 63 20. Total lateral root and lateral root density analysis of overexpression and wild-type lines grown on MS agar plates ............... …………………………. 64 21. Primary root growth in the presence of the DMSO solvent control…………. 66 22. Retention in primary root growth of wild type and transgenic lines when grown on 0.1 µm TIBA relative to the DMSO control at 8 dpg ......... ………. 67 23. Retention in lateral root density of transgenic and wild-type plants when grown on 0.1µm TIBA relative to the DMSO control at 8 dpg.…………… .. 68 24. Primary root growth of the transgenic and wild-type lines when grown on 0.1 µm TIBA ........................................................... …………………………. 69 25. Primary root growth of the transgenic and wild-type lines when grown on 1 µm TIBA .............................................................. …………………………. 70 26. Primary root between the transgenic lines and the wild-type line grown on 0.1µm NPA relative to the DMSO control at 8 dpg …………………………. 71 27. Retention of lateral root density of wild-type and transgenic plants when grown on 0.1µm NPA relative to the DMSO control at 8 dpg ………………. 72 xi 28. Primary root growth on 0.1µm NPA treatment of transgenic lines and wild-type without normalization to the DMSO vehicle control……………… 73 29. Primary root growth of transgenic and wild-type plants when grown on 1.0µm NPA without normalization to the DMSO vehicle control…………… 74 30. Primary root length of all plant lines that were grown on MS, DMSO, 0.1 µm TIBA, 1.0 µm TIBA, 0.1 µm NPA and 1.0 µm NPA………. 75 31. Lateral root numbers of all plant lines that were grown on MS, DMSO, 0.1 µm TIBA and 0.1 µm NPA ................. …………………………. 78 32. Germination percentages of transgenic Arabidopsis and wild-type plants when grown on MS agar for 5 days ........................ …………………………. 81 33. Simplified model of auxin flow into plant root tissues………………………. 94 34. A model for TIBA and NPA and their mode of inhibition on PIN auxin efflux carrier............................................................................................ ……. 96 35. A model of the H+ pump overexpression system along with auxin flow ……. 97 xii 1 INTRODUCTION The Acid Growth Theory describes the fundamental mechanism by which plant cells expand to control growth of the plant (Cleland, 1992; Hager, 2003; Hager, 1991; Rayle and Cleland, 1992a). According to the Acid Growth Theory, the activation of plasma membrane H+-ATPases by auxin causes acidification of the cell wall that, in turn, causes loosening of the cell wall allowing turgor driven cell expansion. The activity of the pump has been proposed to affect many other plant systems and cell functions as well including lateral root initiation, a process in which founder cells are triggered to progress from G2 arrest to re-enter the cell cycle. The purpose of this study is to test 1) whether activation of the pump is able to cause cell expansion as proposed in the Acid Growth Theory and 2) whether activation of the pump is an early signaling event in lateral root initiation. To accomplish this we have overexpressed a constitutively active form of the tomato H+ pump and examined its effects on root growth and lateral root initiation. Overview of H+-ATPase Structure and Function H+-ATPases are thought to participate in numerous physiological functions including: plant defense, phloem loading, stomatal control, regulation of extracellular pH, and in cell growth in response to the hormone auxin (Arango et al., 2003; Pardo and Serrano, 1989).The plasma membrane H+ ATPase utilizes the energy of hydrolysis of ATP to pump H+ out of the cell. Since one H+ is transported outward for each cycle of the pump, its activity contributes directly to the generation of the membrane potential which 2 reaches approximately -200 mV in Arabidopsis (Robertson et al., 2004). In addition to producing charge separation that yields an electrical potential across the plasma membrane, the transport of H+ outward establishes and maintains a chemical gradient for H+ as well. The electrochemical gradient for H+ is often referred to as the proton motive force. The electrochemical gradient for H+ is used to drive a number of secondary active transporters that couple the energy provided by the movement of H+ down their electrochemical gradients to the uphill movement of other solutes. In addition, the membrane potential contributes to the electrochemical gradients that provide the driving force for the movement of charged solutes through ion channels and carriers. As a result of the H+ pumps role in many plant processes, proton pump activity is essential to plant survival (Haruta et al., 2010). Analysis of pump structure can reveal a greater understanding of how the pump itself functions. Cryoelectron microscopy and X-ray crystallography have been utilized to analyze the structure of the plasma membrane H+ ATPase in plants and fungi. The fully functional pump consists of multiple subunits in either a dimeric or a hexameric protein complex (Duby et al., 2009). Each subunit of the pump consists of an approximately 100 kD polypeptide with ten transmembrane -helical domains (M1-10), several cytosolic domains including an actuator (A) domain, a nucleotide binding (N) domain, a phosphorylation (P) domain and a C-terminal cytosolic autoinhibitory regulatory (R) domain (Pedersen et al., 2007). The P domain comprises the ATP binding domain (one on each subunit) which allows for ATP hydrolysis and the phosphorylation event that provides the energy for proton transport. Proposed models place the R domain 3 in the last 100 or so amino acids and this domain then associates with the A domain of the H+-ATPase protein to maintain it in a state of low activity (Ekberg et al., 2010; Palmgren, 1990; Pedersen et al., 2007; Portillo, 2000). Activation of the pump is thought to occur after several phosphorylation events, that cause a conformational change in the tertiary structure of the proton pump, that allows 14-3-3 protein binding to the R domain that activates the pump (Baekgaard et al., 2005; Portillo, 2000). P-type ATPase Superfamily Plant plasma membrane H+-ATPases are members of the P-type ATPase superfamily that are characterized by the formation of a phosphorylated intermediate during transport. The genes making up the P-Type ATPase superfamily have been identified in Arabidopsis and rice whose genomes have been completely sequenced. This has allowed for a total of 46 P-Type ATPase genes to be identified in Arabidopsis and 43 genes in rice (Duby and Boutry, 2009). The P-Type ATPase superfamily includes members found in all organisms from bacteria to archea to eukaryotes and can be broken down into several subfamilies which include Na+/K+ pumps of animals, heavy metal pumps, Ca2+ pumps found in all eukaryotes, and the H+ pumps of plants and fungi. Of this large superfamily, Arabidopsis has twelve genes encoding the plasma membrane H+ ATPase (AHA1- AHA12), nine H+-ATPase genes have been identified in tobacco (PMA1-PMA9), ten in rice (OSA1-OSA10) and at least eight in tomato (LHA1-LHA8), (Arango et al., 2003; Ewing and Bennett, 1994; Ewing et al., 1990; Kalampanayil, 2001; Palmgren, 2001). The relatively large number of H+ ATPase genes highlights its 4 importance in the plant and suggests that there is a need for redundancy to carry out all functions. Auxin Regulation of Plant Growth and Development Activation of the H+ pump by the plant hormone, auxin, is thought to be a key component of the mechanism by which plants regulate growth and development. At the whole plant level, auxins are responsible for controlling the changes in growth that are necessary for phototropism, gravitropism and for controlling overall growth by regulating cell division, cell expansion, and cell differentiation (Woodward and Bartel, 2005). The mechanism by which auxin controls plant growth is not fully understood but is of fundamental importance in plant biology. The current theory that describes how auxin controls plant cell growth is the Acid Growth Theory, whereby auxin causes the efflux of protons out of the cell by activating the plasma membrane H+-ATPase and this acidification causes cell wall loosening which, in turn, allows turgor driven cell expansion (Cleland, 1971; Cosgrove, 2000; Hager, 2003; Hager, 1991). That acidification of the cell wall is able to cause cell expansion is well-supported, thus, elevation in the literature of the Acid Growth Hypothesis to the Acid Growth Theory. Most evidence supports the conclusion that it is activation of the pump that causes this acidification; however, this has not been directly demonstrated. In fact, despite its description as the Acid Growth Theory, this proposed mechanism is not without contention (Kutschera and Niklas, 2007; Kutschera, 1985; Rayle and Cleland, 1992b). 5 Prior research using a variety of techniques has provided much support for the Acid Growth Theory. A brief survey of these studies demonstrates the complexity of auxin control of plant growth along with the depth of acceptance of the theory. First, it is important to note that the Acid Growth Theory, though widely accepted, is still contested by some, as more research is being completed on the subject it is filling in pieces of information that have remained in contention. Some of the first experiments were conducted by Charles Darwin and his father Francis. Using oat coleoptiles they demonstrated that certain stimuli, such as light, cause the plants (coleoptile tips) to bend toward the stimulus and that removal of the tip prevents the response (Berg, 2007; Campbell, 1996). That growth was caused by a diffusible substance was demonstrated by Frits Went who decapitated coleoptiles and replaced the tip with gelatin blocks (Berg, 2007; Campbell, 1996). Coleoptiles whose tips had been removed showed no bending however if the same tips were replaced bending resumed. Gelatin blocks that were incubated with the removed coleoptile tips were allowed to absorb any diffused substance expelled from the tips. These gelatin blocks alone were then shown to stimulate growth when placed on coleoptiles from which the tips had been removed. As studies progressed towards more cellular and molecular approaches from organismal and tissue specific analyses, studies to define the mechanisms that cause cell growth started to become more refined. Many studies had shown that pH changes affect the growth rates in plants (Bonner, 1934). For example, coleoptiles introduced to low pH solutions show increased elongation but when introduced to neutral or basic pH will cease elongation. Studies linking pH along with auxin movement led to the formation of 6 the Acid Growth Hypothesis by David Rayle and Robert Cleland in 1970 and Achim Hager in 1971 (Hager, 1971; Rayle and Cleland, 1970). They first postulated that it was auxin activated elements in the cell membrane that were responsible for cell expansion. They realized that both pH and auxin caused elongation, however, acidic pH caused almost instantaneous elongation while auxin had a lag time between addition of auxin and elongation. This suggested that pH was the mechanism that facilitated elongation and that auxin could affect the pH controlling machinery, which was proposed to be the plasma membrane H+-ATPase. After acidification of the apoplast by the H+ pumps, there are competing hypotheses on the cause of changes in the cell wall. The first is that acidlabile bonds are broken due to acidification and the second is that acidification causes the increase in enzymatic and other machinery (expansins) involved in wall loosening. A range of indirect evidence supports the Acid Growth Theory including an observed decrease in cell wall pH in response to auxin that is thought to result from activation of plasma membrane H+ -ATPase since H+-ATPase inhibitors are able to reduce this response (Rayle and Cleland, 1992a). Studies have tried to determine whether addition of exogenous auxin would cause an increase in the amount of expression of H+ATPases, several of these studies indicated that there was a small detectable increase in expression of mRNA in some instances but no change or a reduction in H+-ATPase mRNA levels was observed in highly auxin responsive tissues such as tomato hypocotyls (Ewing and Bennett, 1994; Frias et al., 1996; Fukuda and Tanaka, 2006). Prior to work in our lab, specific auxin regulated H+-ATPase isoforms have not been clearly identified nor has a direct role for the pumps been clearly demonstrated. There have been implications 7 that the H+ pumps are responsible for acidification of the apoplast by using confocal and light fluorescent microscopy, microelectrode studies and other assays (Nishiyama, 2007; Pitann, 2009; Yu, 2001). Also, studies demonstrated that wall acidification causes loosening of the cell wall structure by activating expansins and glucanases, followed by the dissolving of cell wall elements (Cosgrove, 2005; Kotake et al., 2000; McQueenMason and Cosgrove, 1995). This loosening allows cell expansion driven by turgor pressure in the cell, generated by vacuolar uptake of water (Hager, 2003; Kotake et al., 2000; Rayle and Cleland, 1992a). Auxin may regulate H+ pump activity at multiple levels possibly by regulating its level of transcription, protein degradation, or activity of pumps through phosphorylation of the autoinhibitory domain, by binding of 14-3-3 proteins or by other processes. A range of studies have demonstrated that the pumps are regulated in numerous physiological processes but the mechanism by which auxin regulates the pumps has not been clearly defined in part since key auxin-regulated isoforms have not been identified (Gaxiola et al., 2007). While much still remains to be understood about the mechanisms by which auxin regulates plant growth and development, key pieces of these processes are being uncovered. For example, auxin has been shown to regulate gene expression by regulating the activity of transcription factors that bind auxin responsive promoter elements through ubiquitin-mediated degradation of repressor proteins. Auxin has been shown to regulate the transcription of genes including the SAUR (small auxin-up RNAs), GH3, RolB, and Aux/IAA genes (Gil et al., 1994; Hagen and Guilfoyle, 2002; Maurel et al., 1994). The 8 plasma membranes H+-ATPase, this study’s gene of interest, are among the genes proposed to be regulated by auxin. Evidence for an increase in pump transcription or translation in response to auxin has only been observed in limited studies. One study showed that the amount of immunologically detectable maize isoform MHA2 increased in response to auxin (Frias et al., 1996; Rober-Kleber et al., 2003). Other genes of interest, such as SAURs, are small nuclear proteins that are rapidly degraded. These proteins were first identified in soybeans but are now known to be present in a wide range of plant species including Arabidopsis. Several forms of GH3, which also was initially found in soybeans and are also now known to be present in many species, including Arabidopsis, are auxin responsive (Hagen and Guilfoyle, 2002). GH3s are responsible for conjugating free IAA (Indole-3-acetic acid) with various amino acids by means of IAAamido synthetase and are primarily involved in the regulation of the level of free IAA through its activity in directing conjugation of free IAA to form bound, inactive, IAA. These conjugates are then either degraded or stored (Staswick et al., 2005). The RolB gene from Agrobacterium has also been found to be auxin responsive (Maurel et al., 1994). Since the transcription of these genes increases in response to auxin, it is hypothesized that their promoters include regulatory elements that are responsive to auxin and, in some instances, these have been identified. Among the elements that have been identified are the GH3 AUXREs (auxin response elements) that include the sequence TGTCTC and the sequence ACTTCA from RolB (Mauro, 2001). All of these sequences utilize auxin to modulate gene expression via ARFs, Auxin Response Factors (Guilfoyle and Hagen, 2007). There are numerous activator and repressor ARFs that are 9 found in different levels throughout plant tissues that bind to AUXREs, these are in constant competition with each other to maintain normal plant function. Plant cells regulate binding of ARFs to auxin response elements in a process to control the amount of circulating transcriptional repressors or activators through the ubiquitin pathway. Ubiquination is a highly ordered pathway that is utilized by cells to regulate protein level by tagging proteins which in turn directs them for destruction in the proteosome. Among the proteins that are regulated by ubiquination are the AUX/IAA proteins that are able to bind to ARFs (Hager, 2003; Tiwari et al., 2003). These AUX/IAA proteins are small nuclear proteins that are repressor molecules which bind to both ARFs and to regulatory elements and prevent transcription. Auxins, in high enough concentrations within the cell, can promote the ubiquination of these repressor proteins for rapid degradation by binding with an F-Box subunit, TIR1 and SCF (Skp, Cullin, FBox) to form a ubiquitin ligase complex which catalyzes ubiquination of the repressor proteins. Following ubiquination, the repressor/complex is shuttled to the proteasome for degradation (Mockaitis and Estelle, 2008) allowing essential gene transcription to occur. Among the many other processes that auxin controls, is its ability to control the initiation of lateral roots in plants. Lateral roots arise from the pericycle founder cells in response to auxin and other hormones (Malamy and Ryan, 2001). Studies have suggested that the lateral root founder cells are determined immediately after the cells exit from the apical meristem and that these cells then enter cellular G2 arrest (Dubrovsky et al., 2000). Auxins as well as other plant hormones, cytokinin and gibberellin, are thought to direct re-entry into the cell cycle (Stals and Inze, 2001). In particular, auxin has been shown to 10 downregulate expression of KRP proteins (Kip-related protein) which are known to inhibit cyclin dependent kinases (CDK) which, in turn, are key regulators of the cell cycle (Himanen et al., 2002). Exogenous auxin starvation studies using cultured tobacco cells also prevented cell division from occurring but when it was reintroduced cell division resumed, indicating that it is a necessary initiator of the cell cycle (Rechenmann, 2002). Presumably, with a decrease in the expression of cell cycle repressors in response to auxin, cells may be able to progress through the cell cycle. Previous studies in our laboratory in which 2.3 kb of the LHA2 promoter was fused to GUS, demonstrate that this region of the LHA2 promoter is able to direct transcription very early in lateral root initiation which suggests that the pumps themselves are activated very early in lateral root initiation (Ro, 2000) and, therefore, may play a role in the control of lateral root initiation by auxin. It is early in lateral root initiation that auxin is most important (Dubrovsky et al., 2001), and it is possible that activation of the H+ pumps triggers lateral root initiation. Evidence from other organisms suggests that regulation of the proton pumps by auxin could play a role in controlling the cell cycle. In yeast, proton pumps are responsible for maintaining intracellular pH and have been shown to increase the rate of cellular proliferation with the increased cytosolic pH that results from activation of the pump (Perona and Serrano, 1988). Extensive work in the field of cancer biology has demonstrated that pH changes can cause increased cellular growth and drive progression through the cell cycle. One study using mouse NIH3T3 fibroblast cells found that treatments that are able to increase the H+/Na+ antiporters/exchanger activity increase 11 intracellular pH and seem to cause progression into the cell cycle in tumorogenic fibroblast cells and other transformed cell lines (Harguindey et al., 2005). Another study conducted with transfected mouse fibroblast cells expressing the yeast proton pump, PMA1, showed an increase in cellular proliferation (Perona et al., 1990). If activation of proton pumps by auxin is an essential component in the regulation of the cell cycle in plants, then their activation in the percicycle could be the trigger that drives lateral root initiation. Auxin Transport All plant cells seem to be capable of producing auxin, however production is mainly accomplished in younger rapidly dividing cells and, as a result, a transport system is needed to shuttle the hormone around the plant (Taiz and Zeiger, 2010). The directional, or polar, flow of auxin from root tip towards the root-shoot junction and from the shoot apex downward is largely accomplished by movement of auxin molecules from cell to cell with an apoplastic step between cells while only small amounts of movement is accomplished entirely through the symplast (i.e. from cell to cell through plasmodesmata). Polar auxin transport is a highly ordered process that requires ATP. Auxin uptake is carried out around the whole cell through a combination of auxin influx carrier proteins and diffusion. It has been demonstrated that the plant cell will rearrange the auxin influx and efflux carriers to, in part, produce directional transport of auxin through the cells. Diffusion, in combination with transporters, also contributes to this directional movement. Acidification of the apoplast by the H+-ATPase causes auxin in 12 the apoplast to become protonated. The protonated form of auxin is uncharged (IAAH) and these uncharged molecules are able to freely diffuse through the plasma membrane and so move readily from the apoplast into the cytoplasm. After diffusing into the more basic cytoplasm, they will become unprotonated and the resulting charged form (IAA-) cannot diffuse back across the membrane (Blakeslee et al., 2005) . Anionic forms of IAA (IAA-) are transported through protein carriers including AUX and PIN (Pin-formed) family members. These transporters are H+- coupled secondary transporters, therefore the proton gradient provided by the H+ pump provides the driving force for transport through these carriers. These transporters are often differentially localized in the cell and so produce polar transport as a result of their localization (Li et al., 2005). H+-ATPase Mutagenesis, Overexpression, and Constitutive Activation To be able to understand the roles that the H+ pumps plays in the cell, many studies have utilized expression systems in which H+-ATPase expression has been modified. Several types of studies have been shown that reduction of one isoform of the H+ pump will cause no significant loss of function or organism death (Duby et al., 2009; Haruta et al., 2010). However there are also studies where even a single loss of the H+ pump will cause retardation (Li et al., 2005; Zhao et al., 2000) or organism death. This is true in the case of animal cells where mutation of the Na+/K+ pumps will often lead to cell death. Multiple H+-ATPase pumps which have been silenced or knocked out have also shown severe growth and developmental defects or lethality in plants when multiple isoforms are inactivated (Haruta et al., 2010). As discussed earlier, the number of plasma 13 membrane H+ pump isoforms in plants implies that there may be overlap of function (i.e. functional redundancy) between isoforms. Individual isoforms are expressed in cell and tissue specific patterns that may overlap (Ewing and Bennett, 1994). The apparent redundancy of H+-ATPases may allow cells to provide regulation of the pumps in a more complex pattern than a single or a few isoforms might allow. The regulation of individual isoforms may also allow cells to compensate for experimental manipulations of pump activity. In order to further explore the roles of the H+ pump in vivo, we have overexpressed a tomato H+-ATPase isoform in Arabidopsis. Several other studies have explored the effects of overexpression of the H+ pump on the plant. The overexpression construct used in this study utilizes the 35S Cauliflower Mosaic Virus promoter, which will insure a high degree of expression in all cells (Benfey and Chua, 1990). In order to bypass the effects of the autoinhibitory domain that will generally inhibit the function of the pump without activation, our overexpression construct was designed to include a truncated coding region that yields a pump that lacks the autoinhibitory domain. Other studies have shown that removal of this 38 amino acid residue C-terminal autoinhibitory region leads to activation of the pump (Baekgaard et al., 2005). Overexpression of a constitutively activated pump will help us determine the effects that the H+ pump has on plants. Several studies have overexpressed H+-ATPases in order to define their roles in cell expansion and organogenesis. One study, that is similar in design to this study, overexpressed the tobacco H+ pump PMA4 in the tobacco plant (Gevaudant et al., 2007). This study used the 35S promoter to overexpress a native PMA4 H+ pump and a constitutively activated native PMA4 H+ pump to look at 14 expression, cell expansion and H+-ATPase activity. Overexpression of the native PMA4 increased expression but the plants remained morphological and physiologically identical to the wild-type tobacco, most likely due to the pump autoinhibitory domain, while overexpression of the constitutively activated PMA4 had slightly elevated expression and definitively increased activity of the H+ -ATPase activity compared to the wild-type (Gevaudant et al., 2007). Several overexpression studies using the Nicotiana isoform PMA4 demonstrated changes in expression patterns due to co-suppression of similar pumps (Gevaudant et al., 2007; Zhao et al., 2000). Our study utilizes a H+ pump from tomato (LHA2) introduced into Arabidopsis in order to avoid co-suppression. Cosuppression is the anomaly where introduced genes from the same species can suppress similar endogenous genes and can have a wide range of deleterious effects on the plant, such as silencing of both the transgene and its endogenous copies (Taylor, 1997; Zhao et al., 2000). Co-suppression requires very high sequence similarity; therefore, the utilization of a tomato isoform will reduce the likelihood of co-suppression. However, these genes are still highly conserved and LHA2 is still over 90% identical at the nucleotide level with the most similar Arabidopsis isoforms, so the possibility of cosuppression remains. Plant Plasticity In order to survive under different environmental conditions plants have undergone many evolutionary adaptations. Arabidopsis thaliana is the model species for plant biology and can reveal these adaptations, especially under extreme conditions such 15 as serpentine soils, which are characterized by lower calcium, higher magnesium, and increased amounts of heavy metals while also being nutrient deficient. Studies involving A. thaliana revealed a mutated gene, CAX1, which enables plants to handle the stress of serpentine soils more effectively than wild-type plants. The normal wild-type gene is a tonoplast (vacuolar membrane) calcium proton antiporter, that relies on the proton gradient driven by the vacuolar H+ -ATPases (or pyrophosphatases), and it is normally responsible for transporting calcium into the tonoplast maintaining homeostasis of intracellular calcium. The mutated cax1 gene was found during a screening process in which A. thaliana was grown under simulated serpentine soil and has been shown to be a loss of function mutant (Bradshaw, 2005; Cheng et al., 2003). The studies showed that under low calcium conditions (suboptimal calcium concentrations in soil), mutant A. thaliana with the loss of function cax1 gene will maintain calcium at tolerable levels in the cytoplasm as a result of not being able to transport calcium into the vacuole from the cytoplasm. In natural systems selection will favor changes in genes and how they are regulated that increase survival. In this case mutations have allowed the plant to survive in low levels of calcium by a mutational deactivation of a calcium antiporter, effectively keeping all available calcium in the cytoplasm (Bradshaw, 2005; Cheng et al., 2003). Another member of the Arabidopsis genus, A. lyrata, when grown on serpentine and normal soils were analyzed and revealed several polymorphic differences including many in the P-type heavy metal pumps (Turner et al., 2010). Research using the heavy metal Ptype ATPases, AtHMA4 in yeast, revealed that this pump may allow plants to survive in soils with higher concentrations of heavy metals such as zinc and cadmium (Mills et al., 16 2003; Yang et al., 2005). Serpentine soil experiments using Arabidopsis species have provided a means to determine which genes and P-Type ATPase pumps are expressed under different conditions, which can direct our focus for our current and future studies. Since there are multiple H+ -ATPase genes in plants, it is not surprising that these genes are differentially expressed and can allow the plant to survive and grow in a range of environments. As noted above, plants are resilient but sedentary organisms and have adapted to survive in many environments through cellular modifications and through the ability to modify their growth and development in response to environmental conditions. Several overexpression studies have tried to determine the effects of overexpression of the proton pump on salt tolerance. High saline environments normally cause cellular desiccation as the cells are not able to maintain normal turgor and water levels. In particular to salt studies, overexpression of the vacuolar H+ pumps (which are not P-type ATPases) was found to confer an increase in plant tolerance to high salinity as a result of sequestration of salt in leaves (Gaxiola et al., 2001). The current understanding is that vacuolar H+ ATPase pumps drive the secondary transporters responsible for transport of sugar, ions and other organic acids into the vacuole which is required to maintain internal cellular water balance (Gaxiola et al., 2001). Our overexpression study using the 35S::LHA2 transgene could possibly have similar effects on the plant and promote resistance to a wide range of conditions. 17 Objectives and hypotheses Prior research in this laboratory has been focused on the tomato H+-ATPase isoform, LHA2 including cloning and analysis of the promoter region. The LHA2 promoter region has been shown to contain three GH3 TGTCTC AuxREs and one rolB ACTTCA element ((Idate, 1997; Ro, 2000). To test the possibility that these elements play a role in the regulation of LHA2, Kathy Bradshaw, Manjari Dani, and Greg Gambetta conducted studies in our lab using constructs consisting of 2.3 kb of the LHA2 promoter fused to the reporter gene GUS. These studies demonstrated that this region of the promoter of LHA2 is auxin-responsive throughout plant structures in both Arabidopsis and tomato, including the root, hypocotyl and shoot (Bradshaw, 2001; Gambetta, 2005). Work by Manjari Dani, explored the affect that auxin has on lateral root formation in both tomato and Arabidopsis plants. In her study, the application of exogenous auxins caused a decrease in primary root growth compared to wild-type control plants (Dani, 2007). Numerous other studies have shown these results and this decreased root length has been attributed to auxin levels being driven above optimal levels, with these higher levels causing a decrease in cell growth and cell division (Rahman et al., 2007). To test the role of the H+ pump isoform LHA2, Akihiro Tsuyada and Anna Lee, former graduate students from this lab, successfully prepared a CaMV35S::LHA2 gene construct and transferred it into Arabidopsis using Agrobacterium-mediated transformation and carried out initial studies examining the effect of the overexpression of LHA2 on Arabidopsis growth (Lee, 2007). The CaMV (Cauliflower Mosaic Virus) 18 35S RNA promoter causes constitutive gene expression and has been used in many applications to drive transgene expression to high levels (Benfey and Chua, 1990). The constructs generated in our lab consist of the 35S promoter fused to the LHA2 gene and should cause overexpression of LHA2 upon transfer into Arabidopsis. The prior studies examined two single-copy lines and one multi-copy line. The multi-copy line behaved very differently from the two single-copy lines. This could be due to the effect of a higher level of expression in the multi-copy line, to position effects resulting from where the genes landed in the genome, or some other unknown effect. In order to determine the true effect of overexpression, additional single copy lines were generated and analyzed along with the previously isolated single copy lines. In this study the overexpressing lines are used to test the hypotheses that 1) activation of the plasma membrane H+ pump by auxin causes plant cell growth as described in the Acid Growth Theory and 2) that activation of the plasma membrane H+ pump by auxin triggers lateral root initiation. Since it is possible that overexpression of the pump affects auxin transport we also examined root growth and lateral root initiation in the presence of the auxin transport inhibitors 2,3,5-triiodobenzoic acid (TIBA), and N1-naphthylphthalamic acid (NPA) in order to separate direct effects of activation of the pump from effects on auxin transport. These two chemicals cause a restriction of the polar flow of auxins by affecting auxin efflux carriers enough to limit the influence of auxins on the cell cycle (Casimiro et al., 2001; Estelle, 2001). If we observe increases in root length and/or lateral root initiation in overexpressing lines compared to wild-type lines in the presence of these inhibitors this would provide additional support for the 19 hypotheses that activation of the pump causes cell growth and triggers lateral root initiation. 20 MATERIALS AND METHODS Assembly of the LHA2 Construct The LHA2 construct was prepared prior to this study by previous graduate researchers Anna Lee and Akihiro Tsuyada in the vector pK2GW7 destination vector and designated LHA2/pK2GW7 (Figure 1). This construct consists of an LHA2 cDNA placed downstream of the 35S Cauliflower Mosaic Virus promoter which should drive a high level of expression in all cells. The cDNA was generated using reverse transcriptasePCR and a primer set that was designed to amplify a truncated cDNA with a new stop codon that will yield a polypeptide that is shortened by 38 amino acids relative to the normal LHA2 gene product. This resulted in removal of the autoinhibitory domain and, based on previous studies (Regenberg et al., 1995) yields a constitutively activated pump. Growth of Arabidopsis Plants for Floral Dip Wild type Arabidopsis thaliana Col0 ecotype that were originally obtained from the Arabidopsis Biological Resource Center (http://abrc.osu.edu/) and subsequently propagated by students in our lab were planted on 4 inch diameter plastic pots with soil filled to a mound above the rim of the pot. A nylon mesh cover was secured by rubber band over the top of the mound to allow the shoots and leaves to exit through the mesh and then to not fall out during floral dip. About 15-20 Arabidopsis plants were kept in each pot with adequate spacing between each. The plants were grown for 5-6 weeks in a growth chamber at 25 degrees under 16 hour day and 8 hour night cycles. All wild-type 21 Figure 1. LHA2/p2KGW7 Construct map 22 and transgenic plants were watered daily with the pots sitting in water, to prevent soil desiccation. Inflorescences were periodically cut to promote to increase the number of inflorescences and to cause more uniform flowering. Floral dip infiltration of Agrobacterium tumerfaciens containing the plasmid LHA2/pK2GW7 This is an adapted version of the floral dip method created by Clough in 1998 (Clough and Bent, 1998). Agrobacterium AGL1 cultures containing the plasmid LHA2/pK2GW7 were started 3-4 days prior to infiltration. Starter solutions were used to inoculate 600 ml of LB medium with spectinomycin (55 µg/ml). This culture was allowed to grow until the culture reached 1.8-2.0 at an OD600 which was determined by removing 1 ml samples and measuring the absorbance of these in a Shimadzu Bio-mini DNA/RNA/Protein Analyzer. Cells were spun down at 5000 x g for 10 minutes in a centrifuge. The pellet was re-suspended in an infiltration mixture consisting of 0.5 x Murashige and Skoog Salt and Vitamin Mix (Sigma), 5% w/v sucrose (Sigma), 9.09x10-3 µm of benzylaminopurine (1mg/ml; Sigma), 0.03% v/v Silwet L-77 (Sigma) to a final density of OD600 of 0.8-1.0. Wild type Arabidopsis thaliana, Col0, plants were grown as described above and were used in floral dips when the inflorescences reached approximately 10 cms. The plants were inverted and dipped into the Agrobacterium solution, removed, and placed in an inverted position for an additional 20 minutes. The plants were then removed, placed in an upright position and covered with a plastic dome or plastic wrap to delay evaporation of the solution that remained after dipping. The 23 plants were allowed to continue to grow and mature seeds were collected over the next 45 weeks. M/S agar selection plates Selection was carried out on 0.5X M/S agar plates consisting of MS salt and vitamin solution (final concentration is 0.5x), 1% w/v of sucrose (Sigma), 0.05% w/v of MES buffering agent (Sigma) and 0.8% w/v phytagar (Sigma). The solution was brought up to a pH of 5.8 using a 1N KOH solution, autoclaved for 22 minutes and kanamycin was added to 40 µg/ml after cooling to 65 oC, The plates were poured under sterile conditions and allowed to set overnight. They were stored at 4 oC until needed. Seed Sterilization In order to insure surface sterility during growth, seeds were sterilized before addition to selection plates. The protocol used to sterilize seeds was adapted by previous researchers in this lab. Seeds were placed in separate 1.6ml tubes. One ml of 100% ethanol was added, mixed and allowed to incubate for 5 minutes. The ethanol was removed and one ml of 20% bleach and 0.3% Triton-X100 solution was added, mixed by inversion and allowed to stand for 10 minutes. The bleach solution was removed and one ml of 100% ethanol was added, mixed and incubated for 5 minutes. In a laminar flow hood, ethanol was removed and one ml of 100% ethanol was added and seeds mixed. The ethanol was removed and one ml of sterilized water was added, removed and repeated 3 24 more times. The seeds were then placed at 4 oC for one week to promote even germination. Screening for transformed lines of 35S:LHA2 Arabidopsis Transformed Arabidopsis seeds were grown on MS selection plates (solution described above containing 40 µg/ml kanamycin) for 14 days under sterile conditions. Segregation ratios were used to determine whether the transformed lines contained a single insertion of the 35S:LHA2 gene or multiple insertions. Plants that turned yellow and grew slowly before 10 days were scored as having failed selection and were presumed to lack the transgene. Plants that remained green through the ten day growing period were considered to have the transgene and allowed to self-fertilize for seed collection. A ratio of approximately 3 green (live): 1 yellow (dying) indicates a single insertion of the transgene (or two closely linked copies) whereas a double insertion is expected to produce a ratio of approximately 15 green: 1 yellow with increasing numbers of insertions showing increasing deviation from a 3:1 ratio. Homozygous lines were selected for either single insertion or multiple gene insertions by carrying on lines from which all plated seeds grown on kanamycin yielded only green plants. Seedlings from these plates were moved to soil and seeds were collected. Primer Design All primers were ordered and synthesized through Eurofins, Operon Company (Huntsville, Alabama). Primer design was done using the Invitrogen OligoPerfect 25 Designer and NCBI’s Primer-blast software. Primer sets were designed to have a similar melting point and GC content. Multiple genes were used in this study for both gene verification and real-time PCR see below (Table 1). The primers were designed to span intron and exon sequences in order to differentiate between genomic DNA contamination and cDNA during real-time PCR analysis whenever possible. Verification of LHA2 gene insertion To verify whether the transcript was inserted into the Arabidopsis genome, DNA extractions were performed using Qiagen DNAeasy (Qiagen, Cat.No. 69104) spin columns or a protocol adapted for plant tissue using BioRad’s Instagene Matrix. PCR was performed using primers specific to the LHA2 transgene and GAPDH, mentioned above at a concentration of 10 µm. Each PCR reaction consisted of 1x Finnzymes reaction buffer, 1.25 units of Finnzymes Thermus brockianus Taq Polymerase, 1 µl 2 mM MgCl2 (Finnzymes), 0.1 mM Finnzyme dNTPs, 0.4 µM forward primer, 0.4 µM reverse primer, plus 1 µl (100ng/ µl minimum after genomic extraction) of corresponding genomic DNA and in sterile DNAase and RNAase free water. Typical reaction steps for each PCR were as follows: 1. 5 minutes at 94 degrees Celsius 2. 30 seconds at 95 degrees Celsius for denaturation 3. 30 seconds at 54 degrees Celsius for annealing 4. Cycle repeats 28 times from step 2 5. Last extension is at 72 degrees Celsius for 10 minutes. The resulting DNA was separated by electrophoresis through a 1.5% agarose gel in 1X TAE buffer. DNA was visualized with ethidium bromide or SYBR safe (Invitrogen) staining and an AlphaImager was used to view the gel and capture a gel image. 26 Table 1. Primer sequence data for use in PCR, RT-PCR and qPCR Name LHA2<250bpFPri LHA2<250bpRPri AraRev204CAC AraFor204CAC AraRev119TIP4 AraFor119TIP4 LHA2Rev161 LHA2For161 AraAct2<300bpRPri AraAct2<300bpFpri AraGAPDH831FPri AraGAPDH1089RPri Ara2GAPDH1002FPri Ara2GAPDH1125RPri Length (bp) 20 20 20 20 20 20 23 21 20 20 20 20 20 20 Sequence from 5' to 3' TATTGTGCTTGGTTTTATGC ACTGTCATCATTGCCAAGTA TGTGTTTAATGTTCAGCAT ATCCGTTCAAATCTTTTAT TTCAGTTTCTGTGTCGTAT GAAGCAACATTTCAGTCTC AGTCATCAGTTGCTGTCCTCTG GCCTGATAGTTGGAAGCTGG TCCTGATATCCACATCACA GAGACATCAAGGAGAAGCT GACCAGAAATTCGGTATCAT ACTTCCTCAGCAATGTCTT AACCTCAAAGGAAAACTCAA AGCTCTTTCTCTGCAGAATC 27 Quantification of RNA and cDNA In order to ensure equal loading of RNA and cDNA in reverse transcription and for loading in real time PCR reactions the concentration of isolated DNA and RNA was determined using a Nanodrop ND-1000 instrument. Samples were run in triplicate at the University of California at Davis GBSF building. Verification of LHA2 expression using Reverse Transcriptase-PCR To determine whether the overexpression lines were expressing the LHA2 transgene, reverse transcriptase-PCR was performed. Total RNA from each line, wildType, LHA2-2C, LHA2-3C, LHA2-6H and LHA2-12K was extracted using the RNAeasy Mini Kit with a Qiashredder according to the manufacture instructions. Total RNA was converted into cDNA by reverse transcription with by oligo dT and random hexamer primers using Bio-Rad’s iScript cDNA Synthesis Kit according to the manufacturer’s instructions. These cDNAs were used in PCRs using primers for the LHA2 gene and GAPDH housekeeping gene (see table for LHA2-161 and GAPDH2 primer sets) in 30 cycle reactions. The PCR products were run on a 1.5% TAE agarose gel and visualized under UV light. Quantitative analysis of LHA2 expression Gene expression levels were measured using real time quantitative-PCR on an iCycler iQ5 thermocycler. A 2-step qPCR experiment was used whereby total RNA was converted to cDNA (described above) and then run in a PCR reaction with primers specific for LHA2 or the housekeeping genes TIP 41 and GAPDH. Briefly, 25 ul 28 reactions were prepared using Bio-Rad’s iQ Sybr Green Mastermix according to manufactures specifications. Samples and blanks were performed in triplicate. Cycling conditions were as followed; 1. Activation of the antibody tagged Taq polymerase at 95 degrees for 12 minutes, 2. Denaturation at 95 degrees for 30 seconds 3. Annealing at 56 degrees Celsius for 30 seconds 4. Repeat to cycle 2 for 35 cycles 5. Melt curve analysis for 81 cycles at 0.5 degrees increments for 15 seconds each. Data analysis was completed using Bio-Rad software and data was calibrated to either the wild-type control or the LHA2 overexpression plant with least expression. Phenotypic Analysis Seeds were plated on 0.5X M/S Agar (see above) with 11 seeds per plate and the plates were oriented vertically in a growth chamber at 24 oC with 16h/8h light/dark cycles. Plants were allowed to grow for five days post-germination with germination defined as the time of emergence of the radicle. After five days of growth seedlings were transferred to 0.5X MS plates with either no additions, the solvent DMSO alone 0.005% (volume/volume), or NPA or TIBA at 0 µm, 0.1µm, 1 µm or 10 µm. Primary root lengths and the number of emerged lateral root growth were observed under a stereomicroscope and recorded for 8 consecutive days post germination. The daily measurements were no longer taken for individual plants once plants their roots reached the bottom of the plate while plants were omitted entirely if there was fungal or bacterial contamination or if the root grew in too tight a spiral to allow measurements to be taken. 29 Data for both the root and germination tests were analyzed using Student's t-tests of individual overexpression lines compared to the wild type. Extracellular Acidification Assay Seeds were germinated vertically on M/S plates (described above) for 5 days. Single 5 day old seedlings from wild-type and overexpressing lines, LHA2-2C, LHA23C, LHA2-6H and LHA2-12K were transferred to a well in a 96 Costar black walled fluorescent 96 well plate (Figure 2) containing 250 µl per well of growth solution (0.25x M/S solution, 1% sucrose, 30 µM Dextran Oregon Green MW 10000 (Invitrogen) for 16 hours. Fluorescent emissions were detected at 530 nm and excited at 490 nm using a Promega GloMax microplate reader. The media pH was calibrated using a standard curve range from pH 4.1 to 6.2. 30 Figure 2. A black walled fluorescent plate loaded with plants before obtaining pH fluorescent readings on a Promega Glomax plate reader. Plants were removed prior to reading after growing in solution for 16 hours. 31 RESULTS Agrobacterium-mediated Transformation of Arabidopsis The 35S::LHA2 construct was introduced into the wild-type Arabidopsis thaliana, Col-0 ecotype, by Agrobacterium-mediated transformation using the floral dip method. Approximately 1200 seeds were screened for the presence of the selectable marker that confers kanamycin resistance by growth on 0.5 X MS agar plates containing kanamycin. Following surface sterilization and plating, seeds that are capable of germinating do so and begin to grow. Plants that are resistant to kanamycin thrive and remain green and are selected for further analysis. Plants that are not resistant yellow and die (Figure 3). To determine which seeds contained the transgene, seeds were surface sterilized and plated on kanamycin. The majority of the seeds germinated on kanamycin containing plates within one to three days. After 14 days of growth, nine individual plants remained green and had significant leaf and root growth and, therefore, were selected for further analysis. Plants that were not able to grow on kanamycin and did not have significant root growth turned yellow after 14 days and were presumed to lack the transgene. The nine potential lines, designated LHA2-4 through LHA2-12 that did not yellow was transferred to soil and allowed to self-fertilize for an additional two months for seed collection. The seeds from these nine lines were used to determine the copy number of the LHA2 gene. 32 . Figure 3. Selection on 40 µg/ml kanamycin sulfate (14 days post-germination). An overexpression line, LHA2-12-2, grown on agar plates with kanamycin showing both green (live) and yellow (dead) plants. The number of live and dead plants was used to calculate the segregation ratios which can be used to infer copy number and whether or not the line is homozygous for the transgene. 33 Determination of Transgene Copy Number One of the objectives of this study was to create independent single copy Arabidopsis lines containing the 35S::LHA2 transgene which should lead to the overexpression of the constitutively active plasma membrane H+-ATPase. As described above, in previous work in our lab, Anna Lee and Akihiro Tsuyada generated two overexpressing single copy lines (LHA2-2C and LHA2-3C) and a single multi-copy line (LHA2-1C). While the two single copy lines behaved similarly, the multi-copy line behaved quite differently. Therefore, in this study we set out to isolate additional independent single-copy lines (which are completely independent biological replicates) to determine if the phenotypic effects observed in the two single copy lines are the true effects of H+-ATPase overexpression. Since Arabidopsis is self-pollinating, lines segregating for the presence of the transgene can readily be analyzed to determine the number of transgenes present using the principles of Mendelian inheritance. For example, a plant with a single copy of the transgene (i.e a hemizygous plant) will yield kanamycin resistant progeny in a 3:1 ratio of green to yellow plants since one-half of the progeny will be hemizygous with a single copy of the transgene, one-quarter will be homozygous for the transgene, and one quarter will lack the transgene entirely (Figure 4). A ratio of 15:1 (resistant to non-resistant) is expected for a self-crossed plant with two copies of the transgene (Figure 5). Segregation analysis (Table 2) revealed that two of the lines from the original nine plants, LHA2-6 and LHA2-12 were likely to be single copy lines. The observed segregation ratios of, 1:3.54 for LHA2-6 and 1:3.3 for LHA2-12 follow the Mendelian 34 A a A AA Aa a aA aa Figure 4. Mendelian inheritance in the event of a single transgene insertion. Capital “A” indicates the presence of the transgene at one location in the genome, the lower case “a” indicates the lack of a transgene at that locus. 35 AB Ab aB ab AB AA BB AA Bb aA BB Aa Bb Ab AA Bb AA bb Aa Bb Aa bb aB aA BB aA Bb aa BB aa Ba ab aA bB aA bb aa Bb aa bb Figure 5. Demonstrates the expected outcome of the self-cross of a line containing two copies of the transgene. Capital “A” indicates the presence of the transgene at one location in the genome, the lower case “a” indicates the lack of a transgene at that locus. Capital “B” indicates the presence of the transgene at a second locus and lower case “b” denotes the lack of a transgene at this second locus. The parental genotype following the primary transformation event in the instance of the insertion of two transgenes is then AaBb. Of these progeny, one in fifteen is expected to have the genotype aabb and therefore lack resistance to kanamycin. 36 inheritance pattern expected for a single transgene insertion. A standard Chi square test was used to determine whether segregation ratios were statistically significant to qualify these plants as single copy lines. To be able to accept the null hypothesis, in this instance, that these transgenic lines are not single copy lines, Χ2 values would need to be above a critical value of 3.85, for a P value of 0.05. The 1.852 Χ2 value for LHA2-6 and the 0.0275 Χ2 value for LHA2-12 were below the critical value meaning we can reject the null hypothesis that these lines were “not” single copy. The Chi square test (Table 3) supports the conclusion that these two lines, LHA2-6 and LHA2-12 are indeed single copy lines. With the creation of these two new independent single copy lines and the two homozygous single copy lines previously created by a former researcher in this lab, Anna Lee, the single copy LHA2-2C and the LHA2-3C lines, there are a now a total of four transgenic single-copy lines. The two new independent lines, LHA2-6 and LHA2-12, however would need to undergo further development to ensure they are homozygous lines. Generation of Homozygous Single-copy Lines Once the lines LHA2-6 and LHA2-12 were shown to have resulted from a single transgene insertion event seeds from the F2 generations of each of the parental lines LHA2-6 and LHA2-12 were screened on MS Agar plates with kanamaycin in order to select for individuals that were homozygous for the transgene. Transgenic plants were considered homozygous when all progeny remained green and healthy when grown on kanamycin plates while wild-type plants growing on plates with identical media yellowed 37 Table 2. Segregation analysis for the single insertion lines LHA2-6 and LHA2-12. LHA2-6: Single insertion Observed Expected No. nontransgenic No. transgenic LHA2-12: Single insertion Observed Expected 13 18 28 81 59 54 27.25 81.75 Total sample No. 72 109 Non-transgenic / Tot. sample No. 1/4.54 1/ 4 1/4.3 ¼ Segregation 1:3.54 1:3 1:2.97 1:3 38 Table 3. Chi square (Χ2) test for LHA2-6 and LHA2-12 transgenic lines. Χ2 values (1.852 and 0.0275) are smaller than critical value (3.85) for p=0.05. Observed Expected O-E (O-E)2 (O-E)2/E Nontran 13 18 -5 25 1.389 LHA2-6 Trans 59 54 5 25 0.4630 Total 72 72 1.852 Nontran 28 27.25 -0.75 0.5625 0.0206 LHA2-12 Trans 81 81.75 0.75 0.5625 0.0069 Total 109 109 0.0275 39 and died. Over 100 seeds of each potential transgenic line were grown on kanamycin plates in each screen. Several homozygous F2 plants were identified for each line, designated with a letter (i.e. LHA2-6A, -6B, etc.). All 87 seeds from the plant LHA2-6H that germinated survived on kanamycin as did the 82 seeds from LHA2-12K line. Plants from these screens were transplanted to soil and grown for an additional two months for seed collection to be used in the rest of this study. Several additional homozygous sublines from the primary transformants, LHA2-6 and LHA2-12 were identified and seeds from these plants were also saved for future studies. The two resulting independent homozygous single copy lines (LHA2-6H and 12K) were used in this study. PCR Detection of Transgenes in Extracted Genomic DNA In order to validate the kanamycin screening and the homozygous transformed plants were in fact carrying the LHA2 transgene, genomic DNA extractions were taken from 2 week old whole plants. The DNA extracts from the transgenic LHA2-2C, -3C, 6H and 12K and the wild-type line were then run using PCR to amplify the LHA2 transgene using the LHA2161 primer pair (see primer table, Table 1). The products were separated using on a 1.5% agarose gel to discriminate size of the amplicons. Products that were 161 bp represented the LHA2 transgene. Purified pCR8/LHA2 plasmid DNA was used as the positive control and a 2-log ladder was used as a marker. The results confirm that our transgene is found in the transgenic overexpression lines, lanes 3-6, but not in the wild-type line, lane 2 (Figure 6). 40 Figure 6. PCR detection of the LHA2 transgene in the transgenic overexpression lines and the wild-type parental line. The primer set LHA2-161 was used to determine if the transgene was present. Predicted transgene bands of around 161 bp were observed. Lane 2, the wild-type lane contained no product for the transgene, Lanes 3-6 were LHA2-2C, 3C, 6H and 12K contained a band for the transgene. The positive control was the plasmid pCR8 containing the LHA2 transgene, lane 8. The negative control was a no template control. 41 Detection of LHA2 mRNA: Reverse Transcriptase PCR Before functional and phenotypic root assays were performed it was necessary to determine whether the overexpression lines were actually expressing the LHA2 mRNA transcripts. In order to confirm mRNA expression, reverse transcriptase PCR was utilized. RNA that was extracted from 14 day old transgenic and wild-type plants was quantified to ensure that approximately equal amounts of RNA were used during reverse transcriptase PCR. The resulting cDNA products were then quantified to ensure that equal amounts of cDNA were used in the subsequent PCR. The amplified cDNA products resulting from the reverse transcriptase PCR reactions for LHA2-2C, -3C, -6H and -12K lines and the wild-type line were run on a 1.5% TAE gel using gel electrophoresis to differentiate band sizes. Transgene products were generated using two primer sets, LHA2<250bp and LHA2161 (see primer table) and run in tandem with the housekeeping gene GAPDH (Ara2GAPDH primer set), a loading control and a 2-log ladder size marker. The expected size of the LHA2 bands were 217 bp for the LHA2<250 primer set, 161 bp for the LHA2161 primer set and 123 bp for the GAPDH primer set (Figure 7). The GAPDH primer set yielded Arabidopsis GAPDH at similar levels in all plants, lanes 8-12 (top row) while LHA2 was found to be expressed in the overexpression lines, lanes 3-6 (top row) and lanes 3-6 (bottom row) but not in the wild-type, lane 2 (top and bottom row). 42 Figure 7. Reverse Transcriptase PCR from total RNA from transgenic and wild type Arabidopsis plants. Amplification of LHA2 specific products in lanes 3-7 (top row) and lanes 3-6 (bottom row). GAPDH specific products were run in lanes 8-12, and were used to demonstrate that there was equal RNA loading amongst the transgenic and wild-type lines. 43 Real Time Quantitative PCR Real time PCR was used to verify quantitatively expression levels in the transgenic overexpression lines and to address potential differences between lines. A twostep real time qPCR process was used to determine the mRNA levels. This entailed that the RNA extracted from transgenic and wild-type lines were first converted to cDNA using reverse transcription before running a quantitative qPCR. The RNA and cDNA was quantified at The University of California in Davis using a nanodrop ND-1000, so that even loads could be used during real time qPCR. One µg of RNA was converted to cDNA during the first step of qPCR. Equal concentrations of cDNA were loaded in each reaction vessel for real-time PCR to perform the second step of the real time qPCR. To determine and calibrate expression data, several trial real time PCR reactions were run. A gradient real time PCR was run using the LHA2161, Tip41 and GAPDH primer sets in order to establish what the optimum annealing temperature to the optimum real time PCR product (graphs not shown) was. The optimum temperature for real time PCR using the three primer sets was 56 o C since this providing the best yield of product across all samples. A melting curve established after running the real time PCR determined that there was only one PCR product generated in each reaction (Figure 8). After establishing the optimum temperature for the primer sets a real time PCR was run using cDNA from the transgenic and wild-type lines with the LHA2161, Tip41 and GAPDH primer sets. Using the Pfaffl Method of analysis we are able to determine the relative fold expression level (Figure 9 and Table 4) in conjunction with the primer efficiencies of the three primer sets (Figure 10, 11 and 12). Based on the gradient qPCR and the primer efficiency 44 trials, the gene with the highest level of expression was the Arabidopsis GAPDH gene while the Arabidopsis TIP41 had the lowest level of expression. The LHA2 transgene expression level fell between GAPDH and TIP41 expression. The results also indicated that the expression levels were different across the four transgenic lines, while the wildtype line did not have any product for the transgene (Figure 13). The LHA2 expression data was made relative to the lowest expressing transgenic plant, the LHA2-12K line. Fold expression was calculated between LHA2 after normalization to GAPDH and separately to Tip41, and then averaged together. LHA2-2C had the highest level of LHA2 expression approximately 23 times higher expression than the LHA2-12K line. LHA2-3C had 15 times more and LHA2-6H had 1.1 times greater expression than LHA2-12K. A second complete replicate of the real time PCR assay was performed to confirm the expression levels across the transgenic lines. New RNA extracts were prepared from freshly grown transgenic plants, the results confirmed that expression was indeed lower in the LHA2-6H line and LHA2-12K (Figure 14). The LHA2-6H line had about 2 times expression of the LHA2-12K line and the LHA2-3C line had about 9 times more expression in this replicate. Extracellular Root pH Assay Proton pump activity is the primary contributor to the acidification of the region surrounding the root. If the pump has been successfully overexpressed in an active form, then the plants should acidify the region around the root to a greater extent than wild-type roots. To quantify the ability of plants to acidify the medium, Arabidopsis plants were 45 Figure 8. A melt peak chart run after the gradient temperature analysis. The results confirm that only a single product was created. 46 Fold Expression = (Efficiencytarget)ΔCt( target control-treatment) (Efficiencyreference gene)ΔCt (reference gene control-treatment) Figure 9. The Pfaffl Method of Analysis was used to determine the fold expression of a target mRNA to that of a target gene. 47 Table 4. Pfaffl Method for fold expression. This is an example of how to use the Pfaffl method to determine fold expression of LHA2 using the GAPDH housekeeping gene. The Pfaffl method allows us to take into account primer efficiencies of both LHA2 and GAPDH primer sets. GAPDH Primer Efficiency 94% Efficiency of LHA2161 (Primer Efficiency +1) ΔCt (Control-Treatment) (Efficiencytarget) ÄCt( target control-treatment) Efficeincy of GAPDH (Primer Efficiency +1) ΔCt (Control-Treatment) (Efficiencyreference gene) ΔCt (reference gene control-treatment) Expression = (Efficiencytarget)ΔCt( target control-treatment) (Efficiencyreference gene)ΔCt (reference gene controltreatment) LHA2161 104% 2C 3C 6H 2.04 2.04 2.04 3.94 3.32 -0.72 16.55 10.64 0.60 1.94 1.94 1.94 -0.33 -0.59 -1.07 0.80 0.68 0.49 20.65 15.73 1.21 48 Figure 10. Amplification chart showing the primer efficiency of the GAPDH primer set. A tenfold dilution series was used to determine the primer efficiency. 49 Figure 11. Amplification chart of the primer efficiency for LHA2161 primer set. A tenfold dilution series was used. 50 Figure 12. Amplification chart of the primer efficiency for TIP41 primer set. A tenfold dilution series was used. 51 Figure 13. Analysis of LHA2 mRNA expression using the Pfaffl Method. Fold expression of LHA2 was determined by normalizing the LHA2 levels to GAPDH and TIP41 reference genes. Data was made relative to the transgenic plant with the lowest level of LHA2 expression, LHA2-12K, setting its expression level to one. Error bars represent one standard deviation. 52 Figure 14. Analysis of LHA2 mRNA expression using the Pffafl method. The fold expression was determined using both TIP41 and GAPDH as reference genes. Data was made relative to the transgenic plant with the lowest level of LHA2 expression, LHA212K. 53 first germinated on M/S agar plates and allowed to grow for five days post germination (dpg). These plants were then transplanted into unbuffered MS medium so that only their roots were exposed to the medium. Dextran Oregon Green (Invitrogen), a fluorescent molecule whose fluorescence intensity changes with pH, was used to quantify extracellular pump activity. A pH standard curve was used to calibrate the experimental values. A pH range of 4.1 to 6.2 was constructed to determine which concentration of dextran Oregon Green 488 (Invitrogen) to use (Figure 15). The Oregon Green Concentration that had the best regression value, 30µm at 0.9966, was used for the assay. Using the linear equation from a generated standard curve an extracellular pH can be interpolated from the fluorescent readings of the experimental values (Figure 16). Forty wild-type, eight LHA2-2C, twelve LHA2-3C, fifteen LHA2-6H and twelve LHA2-12K plants were grown for this assay. The transgenic overexpression lines, LHA2-2C had a median pH of 4.73, LHA2-3C had a pH of 4.61, LHA2-6H had a pH of 4.44 and LHA212K had an extracellular pH of 4.42 while the wild-type plants had an average pH of 5.01. A Student’s T-test comparing overexpression lines to the wild-type values were below the p<0.05 threshold (Table 5). The lower pH of the transgenic lines indicates that the pump activity is acidifying the extracellular media at a greater rate than the wild-type. Phenotypic Analysis of Primary Root Growth and Lateral Roots The root acidification results above demonstrate that the pump is overexpressed in roots. Since the overexpression construct is driven by the CMV 35S promoter it is expected to be expressed at similarly high levels throughout the plant. It is also expected 54 2500000 Relative Flourescence Units 2000000 10 um pH y = 282525x - 992429 R² = 0.9937 1500000 20 um pH y = 455805x - 1E+06 R² = 0.9893 30 um pH y = 617997x - 2E+06 R² = 0.9966 1000000 500000 0 0 2 4 6 8 pH Figure 15. Standard curves of pH at different concentrations of dextran Oregon Green. Lines of best fit equation and regression values indicate a high correlation to all pH standard curves. 55 Table 5. Summary of the pH analysis between the overexpression lines and the wild-type. A student’s T-test comparing the transgenic lines to the wild-type was used to determine the p-values. Line WT 2C 3C 6H 12K Average PH 5.01 4.73 4.61 4.44 4.43 Standard Deviation 0.49 0.29 0.32 0.26 0.33 40 8 12 15 13 0.077 0.101 0.093 0.066 0.092 0.023 0.001 5.59E-07 1.82E-05 N Standard Error P values 56 5.2 5 4.8 4.6 4.4 4.2 4 3.8 WT 2C 3C 6H 12K Figure 16. Extracellular pH extrapolated from a 30 µm Oregon Green standard curve. Error bars are in standard error of the mean. LHA2-2C, -3C, -6H and -12K were all statistically significant using a Student’s t-test with p<0.05. 57 to be constitutively active since the coding region was truncated to remove the autoinhibitory domain. Any phenotypic trait acquired due to activation of the pump should be able to be detectable by comparison to the wild-type lines which are not expressing a permanently activated pump. The phenotypic assays used here focused on both primary and lateral roots when grown under normal conditions (0.5 x MS Agar), upright in a growth chamber (Figure 17), and under auxin inhibitory conditions (MS, TIBA and NPA agar or a DMSO vehicle control) to determine effects of overexpression of the pump on root growth and lateral root initiation. Plants were grown for eight days post germination (germination being the start of day 1) and primary root lengths were measured each day (Figure 18). A terminal 8th day graph was made to highlight the differences in primary root lengths (Figure 19 and 30). When transgenic lines were grown on MS plates with no additional treatments, all four single copy homozygous lines, LHA2-2C, LHA2-3C, LHA2-6H and LHA2-12K exhibited longer roots than the control wild-type plants. Average primary root growth for LHA2-2C was 46.6 mm, LHA2-3C was 49.0 mm, LHA2-6H was 45.5 mm and LHA2-12K was 42.3 mm while the wild-type primary root growth was 39.2 mm (Table 6). All results are statistically significant in relation to the wild-type measurements using a Student’s T-test, p<0.01 (Table 6). For lateral root numbers, transgenic and wild-type plants were grown on MS plates for 8 days. On the 8th day lateral root numbers were counted (Figure 20 and 31). Average total number of lateral roots (nLR) of LHA2-2C was 6.46, LHA2-3C was 7.31, LHA2-6H was 5.90 and LHA2-12K was 4.47 while the wild-type was 4.57. Average root density (nLR/Primary Root in cm) for LHA2-2C was 1.39, LHA2-3C was 1.49, LHA2-6H was 58 1.30, LHA2-12K was 1.06 and the wild-type was 1.16. Three of the four transgenic lines, LHA2-2C, LHA2-3C and LHA2-6H exhibited significantly greater total lateral root numbers and lateral root density when compared to the wild-type line using a student’s Ttest, p<0.01 (Table 6) however the results for the LHA2-12K line was not significant. The second part of this root study was to determine the effects of the auxin transport inhibitors, TIBA and NPA on both primary root and lateral root growth. Transgenic and wild-type plants were grown on 0.1µm, 1µm or 10µm of NPA or TIBA or with the vehicle control dosed to the highest concentration NPA or TIBA. 10 µm treatments of either NPA of TIBA were not shown as roots could not be measured. TIBA primary root growth at 0.1 µm (Figure 24) when comparing TIBA treated (DMSO corrected as a percentage) transgenic plants to the wild type indicated that primary root growth decreased in the transgenic lines, at almost a 10% decrease across all of the transgenic lines (Figure 22). Lateral root density was greater in the transgenic lines compared to the wild-type when using normalized data representing, the percent differences between TIBA and DMSO (Figure 23). Primary and lateral root growth on NPA differed from that observed in the presence of TIBA. The comparison of primary root length and the number of lateral roots on 0.1 µM NPA compared to the DMSO controls showed an increase in both the number of lateral roots (Figure 27) and in the length of primary roots (Figure 26) compared to that of the wild type at eight dpg. LHA22C had retained 92.2% of primary root growth, LHA2-3C had 97.2%, lha2-6h had 105.2%, LHA2-12K had 108.7% growth while the wild-type had retained 90.0% primary root growth (Figure 26). Compared to DMSO only controls, plants grown on 0.1 µM 59 NPA retained 51% in LHA2-2C, 48% for LHA2-3C, 45% for LHA2-6H and 45% for LHA2-12K of the number of emerged lateral roots while the number of lateral roots retained in wild-type plants was 7% (Figure 27). Statistical analysis using a two way ANOVA, along with a Bonferroni correction, revealed that the variation within genotype and also within treatment was significant for both primary and lateral roots. A Bonferroni analysis allowed us to look at variation using multiple comparisons between our plant line subsets. For primary roots length and lateral root number and density there were significant differences within genotype between the wild-type and LHA2-2C, LHA2-3C and LHA2-6H while the LHA2-12K line was not significantly different. While they differed from wild-type and LHA2-12K, LHA2-2C, 3C and 12K were not significantly different from each other (Tables 7, 8, 9 and 10). Germination Rates of Transgenic vs. Wild-type Arabidopsis In order to determine whether the transgene caused disruption in germination rates plants were grown for 5 days on MS agar plates with no additional treatment under sterile conditions. Cursory germination rates (Figure 32) were compiled and revealed that the transgenic lines caused an overall decrease in the rates of germination over five days growth. While this could be due to time of harvest of seed or storage conditions these results suggest that overexpression affects seed dormancy or germination. Germination percentages were predominantly lower in the transgenic lines when compared to the wildtype plants however only LHA2-2C and LHA2-12K were significantly lower when using a Students T-test with P<0.05. However the LHA2-3C and LHA2-6H line were 60 approaching significance and with more replicates may potentially reveal a significant effect of overexpression on germination. 61 Figure 17. Transgenic and wild-type plants grown vertically in a growth chamber. Plants were grown for 8 dpg on 0.5X MS, 1 % SUC agar medium. 62 50.0 Growth (mm) 40.0 Wild Type LHA2-2C LHA2-3C LHA2-6H LHA2-12K 30.0 20.0 10.0 0.0 0.0 2.0 4.0 6.0 8.0 Day Figure 18. Growth of transgenic and wild-type lines on MS agar plates. Plants were grown for 8 dpg with root lengths measured daily. A Student’s t-test determined that P<0.05 comparing the transgenic lines to the wild-type line. Error bars are standard error of the mean. 63 Figure 19. Total primary root length at 8 dpg. P values are <0.01 using a Student’s t-test comparing the transgenic lines to the wild-type. Error bars are standard error of the mean. 64 Number of Lateral Roots 8 7 6 5 4 3 Total # Lateral Root Lateral Roots/cm 2 1 0 WT 2c 3c 6h 12k Line Figure 20. Total lateral root and lateral root density analysis of overexpression and wildtype lines grown on MS agar plates. LHA2-2C, 3C and 6H were found to be significant with P<0.05, while the 12K line was not at P < 0.05 using a Student’s t-test. Error bars are in standard error of the mean. 65 Table 6. Lateral and primary root analyses of plants grown on MS agar plates at 8 dpg. In order to determine lateral root density the total number of lateral roots was divided by the primary root length measured in centimeters. A * denotes a p<0.01 and is statistically significant when comparing each transgenic line to the wild-type line using a Student’s ttest. Lateral and Primary Root Analyses WT Average Primary Root 3.92 Length (PR cm) N (Primary Root) 171 Average Total Lateral 4.56 Roots(#LR) Lateral Root Density 1.16 (#LR/PR cm) N (Lateral Root) 125 2c 3c 6h 12k *4.65 *4.90 *4.54 *4.23 37 *6.45 77 *7.31 134 *5.90 98 4.46 *1.38 *1.49 *1.29 1.05 37 77 103 98 66 Primary Root Growth (mm) 60 50 WT 40 2C 30 3C 6H 20 12K 10 0 1 2 3 4 5 6 7 8 Day Figure 21. Primary root growth in the presence of the DMSO solvent control. The data was used to normalize both TIBA and NPA trials. Error bars are in standard error of the mean. 67 100 % growth on TIBA relative to DMSO 90 80 70 60 50 40 30 20 10 0 WT 2C 3C 6H 12K Figure 22. Retention in primary root growth of wild type and transgenic lines when grown on 0.1 µm TIBA relative to the DMSO control at 8 dpg. Error bars are standard error of the mean. 68 % growth on TIBA relative to DMSO 70 60 50 40 30 20 10 0 WT 2C 3C 6H 12K Figure 23. Retention in lateral root density of transgenic and wild-type plants when grown on 0.1µm TIBA relative to the DMSO control at 8 dpg. Error bars are standard error of the mean. 69 50 45 Root Length (mm) 40 Wild Type 35 30 LHA2-2C 25 LHA2-3C 20 LHA2-6H 15 LHA2-12K 10 5 0 1 2 3 4 5 6 7 8 Day Figure 24. Primary root growth of the transgenic and wild-type lines when grown on 0.1 µm TIBA. Error bars are standard of the mean. 70 Primary Root Growth (mm) 35 30 LHA2-2C 25 LHA2-3C 20 LHA2-6H 15 LHA2-12K 10 Wild-type 5 0 1 2 3 4 5 6 7 8 Day Figure 25. Primary root growth of the transgenic and wild-type lines when grown on 1 µm TIBA. Error bars are standard error of the mean. 71 120 % growth on TIBA relative to DMSO 100 80 60 40 20 0 Wild Type LHA2-2C LHA2-3C LHA2-6H LHA2-12K Figure 26. Primary root growth between the transgenic lines and the wild-type line grown on 0.1µm NPA relative to the DMSO control at 8 dpg. Error bars are in standard error of the mean. 72 70 60 % growth on TIBA relative to DMSO 50 40 30 20 10 0 Wild Type LHA2-2C LHA2-3C LHA2-6H LHA2-12K Figure 27. Retention of lateral root density of wild-type and transgenic plants when grown on 0.1µm NPA relative to the DMSO control at 8 dpg. Error bars are standard error of the mean. 73 60 50 WT 40 2C 3C 30 6H 20 12K 10 0 1 2 3 4 5 6 7 8 Figure 28. Primary root growth on 0.1µm NPA treatment of transgenic lines and wildtype without normalization to the DMSO vehicle control. Error bars are standard error of the mean. 74 Figure 29. Primary root growth of transgenic and wild-type plants when grown on 1.0µm NPA without normalization to the DMSO vehicle control. Error bars are standard error of the mean. 75 Figure 30. Primary root length of all plant lines that were grown on MS, DMSO, 0.1 µm TIBA, 1.0 µm TIBA, 0.1 µm NPA and 1.0 µm NPA. Absolute primary root length was gathered at eight days post germination. 76 Table 7. Two-way ANOVA on primary root length. The two-way ANOVA was used to determine if there was a significant difference between genotype and treatment groups. Type III Sum of Source Squares df Mean Square F Sig. Corrected Model 48634.761a 29 1677.061 19.070 .000 Intercept 665821.193 1 665821.193 7571.146 .000 Treatment 32982.955 5 6596.591 75.011 .000 Genotype 3339.780 4 834.945 9.494 .000 treatment * genotype 5105.654 20 255.283 2.903 .000 Error 86710.744 986 87.942 Total 1835092.250 1016 135345.506 1015 Corrected Total a. R Squared = .359 (Adjusted R Squared = .340) 77 Table 8. Post Hoc Bonferroni analysis of the genotype for the primary roots. Examination of the variation between the wild-type lines and the overexpression lines revealed that the LHA2-2C, LHA2-3c and the LHA2-6H lines were significantly different than the wild-type however the LHA2-12K overexpression line was not significantly different. (I) 95% Confidence Interval genotype (J) genotype Mean Difference (I-J) Std. Error Wild 2c -5.7298* 1.19680 .000 -9.0969 -2.3628 3c -5.1694* .87945 .000 -7.6436 -2.6952 6h -4.1523* .80572 .000 -6.4191 -1.8855 12k 1.4636 .84322 .829 -.9087 3.8359 Wild 5.7298* 1.19680 .000 2.3628 9.0969 3c .5604 1.27487 1.000 -3.0262 4.1471 6h 1.5776 1.22517 1.000 -1.8693 5.0244 12k 7.1934* 1.25015 .000 3.6763 10.7106 Wild 5.1694* .87945 .000 2.6952 7.6436 2c -.5604 1.27487 1.000 -4.1471 3.0262 6h 1.0171 .91768 1.000 -1.5647 3.5989 12k 6.6330* .95077 .000 3.9581 9.3079 Wild 4.1523* .80572 .000 1.8855 6.4191 2c -1.5776 1.22517 1.000 -5.0244 1.8693 3c -1.0171 .91768 1.000 -3.5989 1.5647 12k 5.6159* .88302 .000 3.1316 8.1001 Wild -1.4636 .84322 .829 -3.8359 .9087 2c -7.1934* 1.25015 .000 -10.7106 -3.6763 3c -6.6330* .95077 .000 -9.3079 -3.9581 6h -5.6159* .88302 .000 -8.1001 -3.1316 2c 3c 6h 12k Based on observed means. The error term is Mean Square (Error) = 87.942. *. The mean difference is significant at the .05 level. Sig. Lower Bound Upper Bound 78 Figure 31. Lateral root numbers of all plant lines that were grown on MS, DMSO, 0.1 µm TIBA and 0.1 µm NPA. Absolute lateral root numbers were gathered at eight days post germination. 79 Table 9. Two-way ANOVA on lateral roots. Type III Sum of Source Squares df Mean Square F Sig. 2184.143a 19 114.955 11.499 .000 4305.135 1 4305.135 430.629 .000 Treatment 951.407 3 317.136 31.722 .000 Genotype 210.073 4 52.518 5.253 .000 treatment * genotype 254.363 12 21.197 2.120 .014 Error 7108.087 711 9.997 Total 28020.000 731 9292.230 730 Corrected Model Intercept Corrected Total a. R Squared = .235 (Adjusted R Squared = .215) 80 Table 10. Post Hoc Bonferroni analysis of the genotype for the lateral roots. Examination of the variation between the wild-type lines and the overexpression lines revealed that the LHA2-2C, LHA2-3c and the LHA2-6H lines were significantly different than the wild-type however the LHA2-12K overexpression line was not significantly different. 95% Confidence Interval (I) genotype Wild 2c 3c 6h 12k (J) genotype Mean Difference (I-J) Std. Error Sig. Lower Bound Upper Bound 2c -2.2076* .47235 .000 -3.5377 -.8775 3c -1.7393* .34736 .000 -2.7174 -.7612 6h -1.3053* .32894 .001 -2.2315 -.3791 12k .3411 .33708 1.000 -.6081 1.2903 wild 2.2076* .47235 .000 .8775 3.5377 3c .4683 .49222 1.000 -.9177 1.8543 6h .9023 .47940 .602 -.4476 2.2522 12k 2.5487* .48502 .000 1.1830 3.9144 wild 1.7393* .34736 .000 .7612 2.7174 2c -.4683 .49222 1.000 -1.8543 .9177 6h .4340 .35689 1.000 -.5709 1.4389 12k 2.0804* .36440 .000 1.0543 3.1065 wild 1.3053* .32894 .001 .3791 2.2315 2c -.9023 .47940 .602 -2.2522 .4476 3c -.4340 .35689 1.000 -1.4389 .5709 12k 1.6464* .34689 .000 .6696 2.6232 wild -.3411 .33708 1.000 -1.2903 .6081 2c -2.5487* .48502 .000 -3.9144 -1.1830 3c -2.0804* .36440 .000 -3.1065 -1.0543 6h -1.6464* .34689 .000 -2.6232 -.6696 Based on observed means. The error term is Mean Square (Error) = 9.997. *. The mean difference is significant at the .05 level. 81 % Germination Germination Percentage 100 90 80 70 60 50 40 30 20 10 0 WT 2c 3c 6h 12k Line Figure 32. Germination percentages of transgenic Arabidopsis and wild-type plants when grown on MS agar for 5 days. A student’s T-test for LHA2-2C and LHA2-12K germination percentage were significant, p<0.05 when compared to the wild type. LHA23C and LHA2-6H were approaching significance. 82 DISCUSSION While this study’s ultimate purpose was to define the function of the H+ pump, it was important to consider the possibility that overexpression of the pumps had caused the unexpected changes in our transgenic plants. As is the case in most biological systems, H+ pumps are controlled by multiple layers of regulatory mechanisms and in this study we attempted to artificially circumvent these. Two independent single copy Arabidopsis lines were generated (LHA2-6H and LHA2-12K) which are overexpressing a truncated cDNA encoding the tomato plasma membrane H+-ATPase isoform LHA2. LHA2-6H and LHA2-12K were analyzed alongside the two existing independent single-copy lines LHA2-2C and LHA2-3C originally generated by Anna Lee. The LHA2 cDNA was truncated as a means to avoid auto regulation by the autoinhibitory domain located in the c-terminus of the functional protein. Several studies have indicated that auxin effects on regulatory proteins such as 14-3-3 can modulate the activity of the pump by enhancing phosphorylation events of the autoinhibitory domain and establish activation (Arango et al., 2003; Darginaviciene, 2008; Ekberg et al., 2010). This enables the cell to modify endogenous pump activity based on cellular needs. Removal of this domain has been shown to confer permanent and stable activation in yeast and in other plants while maintaining protein function (Gevaudant et al., 2007; Regenberg et al., 1995). The truncated H+ pump in our overexpression construct was expected to be released from regulation that was conferred by the autoinhibitory region. The LHA2 coding region (without the autoinhibitory region), was artificially fused to the 35S promoter in order to 83 direct a high level of expression in all cells. Previous overexpression studies have achieved high mRNA levels in plants using the 35S CaMV promoter for transgene expression (Gevaudant et al., 2007; Li and Steffens, 2002). Unlike the natural LHA2 promoter that confers cell specific regulation and is responsive to multiple internal cellular signals, the 35S promoter is not dependent on these organism specific cellular signals and maintains constitutive levels of expression of its downstream gene. We examined the effects of the overexpression of the 35S::LHA2 construct and determined that the transgenic plants were all expressing the LHA2 gene. As is typical in overexpression analyses such as these, while all lines expressed the transgene, both qPCR and semi-qPCR demonstrated that the level of expression varied across lines. These results demonstrated that all lines were overexpressing LHA2 at the mRNA level as a result of the presence of the transgene. Of the two housekeeping genes we have used for Arabidopsis expression analysis, GAPDH (glyceraldehyde-3phosphate dehydrogenase) was expressed at a high level throughout all plants (transgenic plants and wild-type) while TIP41 (Tip-like protein) was expressed at a lower level. LHA2 expression in the transgenic plants was less than GAPDH for all lines and greater than TIP41 for LHA2-2C and LHA2-3C while LHA2-6H and LHA2-12K express LHA2 at a level similar to TIP41. The difference in absolute levels of expression between transgenic lines is likely due to positional effects of insertion of the transgene into the genome. For instance, in the LHA2-2C and LHA2-3C lines, if gene construct landed in areas of the genome that were undergoing greater amounts of transcription while in 84 LHA2-6H and LHA2-12K lines, the construct inserted in an area of lower transcription. This could explain why there is a difference in expression between overexpressing lines (Harmon and Sedat, 2005). As is the case in prior studies, high expression normally resulted in increased amounts of protein as was the case with poly-phenol oxidase overexpression in studies aimed at understanding plant defense (Li and Steffens, 2002) or with the AVP1 tonoplast H+-ATPases (Gaxiola et al., 2001) and plasma membrane H+ pumps, PMA4 (Gevaudant et al., 2007). Even though artificially overexpressed plants can lead to an increase in gene expression, it does not necessarily translate to more protein production in every case. For instance, transgenic plants with natively derived transgenes that are artificially expressed can potentially result in RNA-DNA and RNA-RNA, binding of complementary RNA sequences which are then degraded thereby reducing the amount of circulating mRNA (Taylor, 1997). In most cases, this gene silencing feedback mechanism occurs when high levels of transcript are present and any effects that could have been caused by the transgene are muted or masked by regulatory co-suppression. We selected the non-native tomato H+-ATPase LHA2 for overexpression in Arabidopsis in order to decrease the likelihood of co-suppression. The expression levels that we observed support the conclusion that co-suppression is negligible or is not occurring since all lines showed significant levels of LHA2 expression. We did not examine mRNA expression levels of Arabidopsis H+ pump isoforms to determine if the native pumps were down-regulated, however, the root acidification assays performed here demonstrate that total proton 85 pumping increased in overexpressing lines which suggests that Arabidopsis isoform expression was not reduced due to co-suppression or any loss was compensated. Housekeeping genes in qPCR Typically in qPCR, a gene (or set of genes) is chosen against which the expression of the gene of interest will be normalized and, importantly, the expression of this gene should be constant across treatments examined. Other studies demonstrated that the expression of the GAPDH and TIP41 housekeeping genes in Arabidopsis is relatively stable and therefore are appropriate for use in expression studies (Exposito-Rodriguez et al., 2008; Schmittgen and Zakrajsek, 2000). We used two housekeeping genes, GAPDH and TIP41, as internal controls in order to reduce the effects of variation in expression that might result from normalizing expression to that of just a single housekeeping gene. In this study GAPDH and TIP41 were expressed at similar levels relative to each other across all lines (wild-type and overexpressing) which provides support that these are good internal controls upon which to base the expression of the LHA2 transgene. Optimum range of biological systems Biological systems work in an optimum range of environmental factors from pH to salt balance, plant systems are no different when it comes to how its cells react to changing to environments. In our study, we have manipulated the proton pumps through overexpression of the pumps and auxin flow through the use of transport inhibitors. This created a permanently activated H+ pump extruding protons into the apoplast, as long as 86 there is available ATP, responsible for acidification and for the electrochemical motive force to drive secondary transporters. Auxin flow regulators are another artificial means for us to impose changes on a plant system by effecting auxin availability. Plant systems operate at or near physiological optimum conditions like the auxin system (Casimiro et al., 2001). Any subsequent shift in conditions may result in significant changes within a plant. For instance, if exogenous auxin is added to the root system, we see a phenotype shift resulting in an increase in the number of lateral roots but a decrease in primary root length (Casimiro et al., 2001; Dani, 2007). Our work here examining both primary and lateral roots supports previous claims about acid growth and lateral root development and that there is an organized balance between auxin and pH systems in plant growth. Therefore, by manipulating the H+ pumps and auxin system by using auxin transport inhibitors, we are able to observe at a more basic level, in roots, the growth of cells and entry into the cell cycle. The role of the H+ pump in cell elongation One of the major roles of the plasma membrane H+ pumps in plants is to create the membrane potential and proton gradients that are used to drive secondary transporters. In addition, changes in intracellular and extracellular pH caused by regulation of the activity of the pump are also thought to be of central importance in the regulation of cell expansion as described in the Acid Growth Theory. Our results provide support for this proposed role since overexpression of the pump resulted in increased growth in primary roots. It showed that the four overexpression lines (LHA2-2C, -3C, - 87 6H and -12K) had increased primary root growth, (LHA2-2C was 46.6 mm, LHA2-3C was 49.0 mm, LHA2-6H was 45.5 mm, LHA2-12K was 42.3 mm while the wild-type was 39.2 mm) when grown on normal MS agar plates, strongly supporting the key element of the Acid Growth Theory that an activated pump is directly responsible for proton extrusion into the apoplast thereby allowing for cell expansion. Apoplast acidification either directly causes cell wall loosening events or activity of the H+ pumps will enable and maintain enzymes, exo- and endo-glucanases and expansin molecules, to break down covalent and hydrogen bonds and loosen the cell wall cellulose and polysaccharide elements to allow for turgor driven cell elongation. (Cosgrove, 2005; Kotake et al., 2000; McQueen-Mason and Cosgrove, 1995). Our study did not determine whether this increased growth was indeed due to an increase in cell expansion or, as might also account for an increased root length, an increase in cell division. Nonetheless, if the pump functions as described in the Acid Growth Theory, overexpression should cause an increase in root length. To date mostly indirect evidence for the Acid Growth Theory exists despite its elevation from “Acid Growth Hypothesis” to “Acid Growth Theory” in many instances. This study directly supports the Acid Growth Theory of cell elongation by providing a direct means to test acid growth, by overexpressing an activated H+ pump without the need for auxin activation, a means to determine if the H+ pumps are expressed, RTqPCR, and to determine if the H+ pumps are active and readily effecting the cell, e.g. extracellular fluorescent pH assay to directly demonstrate activity, while the root phenotype assays will provide evidence for cell elongation. Since we saw an increase in 88 primary root growth and the only difference between the wild-type and the overexpression lines was the gene construct, the differences observed between the primary root growth of the overexpression lines can be attributed to the H+ pump. Our study also used the auxin flow regulators TIBA and NPA to isolate auxins effects on the endogenous H+ pumps so we can begin to focus on the H+ pump activity from the non-native overexpression H+ pumps. Our results show that auxin flow was most likely affected by auxin transport inhibitors and not necessarily the H+ pumps were causing significant changes in auxin flow since we had a decrease in lateral roots from DMSO controls to results on both TIBA and NPA. Had auxin flow not been affected, we would not have seen a drop in retention in the lateral roots. We did see a difference in our TIBA and NPA primary root results but this may have been due to a difference in the modes of inhibition between these chemicals which will be discussed later in this discussion. Since the MS and NPA data shows that all transgenic lines had greater primary root growth retention than the wild-type, this supports our hypothesis that activation of the plasma membrane H+ pump by auxin causes plant cell growth as described in the Acid Growth Theory. The TIBA results, on the other hand, show an effect that is opposite to what we predicted since we expected that TIBA would have caused a similar effect to the NPA experiments but instead the transgenic lines had less primary root growth retention comparing the transgenic lines to the wild-type line. 89 The role of the H+ pump in cell cycle regulation The second area that this study focused on was the hypothesis that changes in cytosolic pH resulting from activation of the H+ pump might contribute to regulation of the cell cycle and, as a result, promote lateral root initiation. Prior research in our laboratory using LHA2::GUS reporter gene analysis in Arabidopsis and tomato indicated that the promoter directs expression very early in lateral root formation. Our previous analysis of single-copy overexpression lines indicated that there was a general increase in the number and density of lateral roots. As a result, we were careful to examine this phenotype in the current study. The main difference between this study and other overexpression studies is that we used a non-native plasma membrane H+ pump transgene from tomato, LHA2 and inserted it into Arabidopsis thaliana. As suggested earlier, this addressed the issues with co-suppression and regulation that others studies may have witnessed. The results here suggest that lateral root initiation can be separated from the effects of auxin transport and hence the endogenous H+ pumps through auxin transport inhibitors while activity from the H+ pump alone is able to cause the initiation of lateral roots. Several theories surround the initiation of lateral roots. This includes the G2 cell cycle arrest theory, where all pericycle cells leaving the apical meristem enter a period of non-replication by exiting the cell cycle at G2 and must de-differentiate and re-enter the cell cycle to complete lateral root initiation (Blakely, 1982). The other theory is that the pericycle cells never leave the cell cycle after moving from the apical meristem, only stopping at G1 phase and with the right trigger will progress through the cell cycle. 90 Although this was found to only be typified by young lateral roots as older root pericycle tissues are stalled in G2 further up the root and still able to develop into lateral roots (Beeckman et al., 2001; Dubrovsky et al., 2000). Lateral root phenotypes in the present study when grown on MS medium indicated that 3 out of the 4 overexpression lines, LHA2-2C, LHA2-3C and LHA2-6H all had significantly increased lateral root densities at 1.39 LR/cm (# lateral roots/ cm of primary root), 1.49 LR/cm, and 1.30 LR/cm respectively while the wild-type plants had lower lateral root density at 1.16 LR/cm. This strongly suggests that the proton pump is involved in lateral root ontogeny. Another contributing factor in lateral root development is auxin. Auxin has been shown to be required for the initial cell divisions in lateral root organogenesis; research excising young lateral root primordia found that cell division stopped and did not progress until exogenous auxin was added to the system. This was also supported in alfalfa protoplast studies although a direct mechanism has not been found (Casimiro et al., 2001; Pasternak et al., 2002). The results of this study suggest that activation of the H+ pump and the resulting change in cytosolic and/or apoplastic pH may cause the reentry or progression of pericycle cells into the cell cycle and that this drives lateral root initiation. This coupled with our previous LHA2::GUS analysis provides support for the conclusion that, in tomato, activation of LHA2 by auxin drives lateral root initiation. It also supports lateral root initiation hypothesis that auxin acts to stimulate lateral root initiation by activation the H+ pump, which then triggers progression through the cell cycle beginning the development of a new organ. 91 As discussed earlier, yeast and mouse fibroblast studies have shown that when a proton pump was expressed in these systems that there was an increase in cell proliferation as intracellular pH became alkaline, which suggests that cell division and proliferation is dependent on changes in cytoplasmic pH in other eukaryotic systems (Harguindey et al., 2005; Perona and Serrano, 1988). Only a couple of studies have tried to explain pH and cell cycle regulation in plants. A plant study using Bidens pilosa, Spanish Needle, explored alkalinization events further in the cytoplasm and discovered that during cell division transient fluxes in cytoplasmic pH occurred (Pichon, 1994). A protoplast study in plants using cells from alfalfa determined that there were increases in cytoplasmic pH during cell activation and division (Pasternak et al., 2002). Both these studies along with our own research indicate potential for regulation of the H+ pump to be the mechanism by which transient cytosolic pH changes may be generated to control the cell cycle. The function of the H+ pump to move protons out of cytoplasm into the apoplast led us to support the hypothesis initially proposed by Pichon and colleagues. They believed that the H+ pump is able to cause this effect, transient cytoplasmic alkalinization demonstrated in the overexpression lines in this study. It is important to note that exogenous auxin added to roots increase the lateral root density similar to this study perhaps suggesting the mechanism that auxin plays in lateral root development is activation of the H+ pump and subsequent alkalinization triggers lateral root initiation. We cannot rule out the possibility that perhaps the overexpression of the H+ pump is increasing auxin flow through increased diffusion of protonated IAA and that this increase is the cause of the increase in lateral root initiation. As mentioned earlier, auxin 92 has been specifically implicated in the development of lateral roots and complete removal of auxin will cause the cessation of cell division (Himanen et al., 2002). All cells are capable of producing auxin so a basal level may be maintained for nominal cell function while use of the auxin transport inhibitors will severely diminish auxin flow between cells and allow us to isolate the H+ pump from the effects of increased auxin transport potentially due to overexpression. Our extracellular pH assay and lateral root density data comparing the wild-type and transgenic plants also supports our second hypothesis that activation of the pump is an early signaling event in lateral root initiation and suggests that pH changes caused by activation of the pump are sufficient to trigger the initiation of lateral roots. Both the results using the auxin transport inhibitors NPA and TIBA suggested that the overexpression of the H+ pumps caused a greater percentage of lateral root density with the overexpression lines compared to the wild-type. Combined with the data for the primary root growth, this could explain that we have inhibited auxin flow using the receptor based NPA and the H+ pumps are able to trigger lateral roots and increase primary root growth in transgenic lines due to function of the H+ pump, our second hypothesis. It is necessary to take a more in depth approach to look at auxin flow to determine whether there is a mechanistic change polar auxin flow or whether results are due to function of the pump triggering lateral roots. 93 Auxin flow dynamics All plants cells are capable of producing auxin, however; most production occurs in rapidly dividing cells and tissue like shoot apical meristems and leaves. Therefore there is a need to transport auxin to distant tissues which produce small quantities of auxin including the roots. This long distance movement of manufactured auxin toward the root tip is accomplished through phloem sieve-tube elements in the vascular cylinder after which root polar auxin flow back up the root toward the root-shoot junction is maintained by transport through nonvascular tissue of the cortex. We tried to separate the effects of auxin flow through cells by adding auxin transport inhibitors to isolate the transgenic plants with the overexpression H+ pumps from the wild-type endogenous H+ pumps (Figure 33). We had used the auxin transport inhibitors NPA and TIBA to determine the effects of these chemicals had specifically on plant roots, whether they changed growth patterns and we could examine the transgenic pump in absence of endogenous pump activity. NPA is an effecter of plant auxin efflux carriers effectively sequestering auxin, - IAA, inside the cells and at the tissue level, in the root tip. Studies show that NPA and plant flavonols bind plasma membrane bound flavanoid receptors in the plant cell that has the ability to shut down the activity of the efflux carriers such as the PIN-formed family of proteins preventing auxin transport and sequestration within the cell and surrounding tissue (Figure 34) (Murphy et al., 2000; Peer et al., 2004). TIBA, the other auxin flow regulator used in this study, is structurally similar to auxin, and is thought to compete with auxin for binding with the auxin efflux carriers to be transported out of the 94 Figure 33. Simplified model of auxin flow into plant root tissues. Auxin is transported through vascular tissue to the root tip, after which auxin is transported through cells back up the root. TIBA and NPA work to effectively sequester auxin in the root tip. 95 cell (Casimiro et al., 2001; Peer et al., 2004; Wu, 2007). Since TIBA is a competitive inhibitor; it can prevent or severely hinder auxin flow by competing with the circulating auxin to be extruded from the cell at the auxin efflux carrier (Figure 34). Why we saw decreased primary roots in overexpression lines compared to the wild-type is that we may see a marked increased amount of diffusion of protonated IAA that has entered the cell due to activity of the transgenic H+ pump. This is due to the nature of our gene construct, as mentioned earlier; the transgene H+ pump was designed so that it is always activated compared to a normal endogenous H+ pump which is self-regulated. The gene construct also conferred constitutive expression by way of the 35s promoter meaning that there should be a high amount of the transgenic H+ pump proteins in the cell. Both amount of transgene and activity level may translate to a marked decrease in pH of the apoplast which in turn may lead to increased diffusion of protonated IAA into the cytoplasm. Another possibile interpretation for the TIBA results is that if the organism were to increase auxin production to be transported to auxin deficient areas, then this may allow auxin flow to resume because TIBA is a competitive inhibitor. As long as either TIBA or auxin concentrations are high enough, significant changes in auxin flow may occur. However, it could also be another compensatory mechanism that is affecting primary root growth with TIBA. The consensus of various studies have indicated that the use of TIBA and NPA on roots will cause a decrease in the overall growth rates and length of primary roots (An, 2001). We believed that a potential artifact of overexpression of the H+ pumps would be a greater amount of IAA to be transported through diffusion or transport systems compared to the wild-type plants (Figure 35). Our hypothesis postulates that 96 Figure 34. A model for TIBA and NPA and their mode of inhibition on PIN auxin efflux carrier. TIBA works through competitive inhibition with auxin and is concentration dependent. NPA works through non-competitive inhibition and is receptor based. 97 Figure 35. A model of the H+ pump overexpression system along with auxin flow. The overexpression system (right) may lead to decreased pH compared to a wild-type system (left) due to quantity and activity of the pumps. The overexpression system (right) can lead to an increased rate of diffusion of IAA into the cell which may in turn effect TIBA competitive inhibition. 98 activation of the pump causes growth according to the acid growth theory and would result in greater primary root and lateral root growth of the overexpression lines even in the presence of auxin transport inhibitors. The TIBA root phenotype results indicated that we had diminished primary root growth but increased numbers of lateral roots in transgenic plants compared to the wildtype. We had predicted that the overexpression plants would have greater primary root lengths compared to the wild-type plants when grown on TIBA, however, our results showed the opposite effect. Looking at the lateral roots of plants grown on TIBA, our results indicated that the wild-type line had lower numbers of lateral roots when grown under auxin inhibitor constraints than the transgenic lines and significantly lower than when the wild-type plants were grown on normal MS or DMSO media. This implies that auxin flow is, in fact, being inhibited. In prior research in this laboratory and other studies as well, the addition of exogenous auxin decreased the growth of the primary root while also increased the number of lateral roots in the transgenic lines compared to wild-type (An, 2001). Since we are trying to competitively limit auxin transport through TIBA via auxin efflux proteins, an increase in production at the organismal level for instance, due to low levels of circulating auxins, could have overwhelmed competitive inhibition leading to diminished primary root growth in our overexpression lines. For NPA, we proposed that the overexpression and function of the H+ pump, extruding protons across the membrane into the apoplast, would cause alkalinization of the cytoplasm and acidification of the apoplast that would lead to both a greater primary root length (i.e. greater percentage in primary root) and greater number of lateral roots 99 comparing our transgenic lines to our wild-type. Several studies that looked at plant root growth under NPA conditions can provide insight into our study. Casimiro and colleagues studied the effects of NPA on plant roots (Casimiro et al, 2001). They found that as concentrations of NPA were increased various stages of lateral roots were prevented from progressing to form full lateral root primordia, demonstrating the effect of auxin inhibition on plant roots. This was also supported when the roots were added to plates with exogenous auxin since the plants continued with normal lateral root initiation (Casimiro et al., 2003; Casimiro et al., 2001). This study demonstrated greater primary root length and lateral root density of plants grown on M/S, DMSO and NPA (TIBA with just lateral roots) in transgenic lines compared to wild-type plants. These results support both of our hypotheses, 1) that activation of H+ pumps will cause cell growth as suggested in the Acid Growth Theory in primary roots and also 2) cause an increase in the initiation of lateral roots. The primary and lateral root growth experiments demonstrated that even in the presence of auxin transport inhibitors overexpression and activity of the H+ pump was causing an effect in plant growth and lateral root initiation. Our results, even though the TIBA results were not expected, demonstrate that activity of the pump is a driving factor in primary root growth and an important factor in lateral root development. Activity Evidence of a pH effect of the H+ pumps can be directly supported by our extracellular pH activity assays where we observed a significant decrease in the pH of 100 medium comparing the transgenic plants to the wild-type plants. We attribute this difference between wild-type and transgenic lines to a decrease in pH caused by the activity of the transgenic H+ pumps acidifying the medium. While this decrease does not directly demonstrate a concomitant alkalinization of the cytoplasm, it does show the capabilities of the pump to maintain or cause pH changes in plant cells and surrounding cell tissue and environment. Separated from the effects of auxin, by auxin transport inhibitors, our results suggest that the H+ pumps cause cell growth (primary root) due to pump activity and cytoplasmic pH can initiate re-entry into the cell cycle (lateral roots) and even in the absence of auxin flow thereby supporting our first hypothesis that activation of the pump can lead to cell growth as stated by the Acid Growth Theory and our second hypothesis, that activation of the pump is an early trigger in lateral root initiation. Conclusions The purpose of this study was to answer the question of whether activation of the pumps causes cell growth according to the Acid Growth and can cause cell cycle progression in order to define the function of the pump in plant growth and organogenesis. We have successfully used a non-native overexpressed and permanently activated pump to explore whether we would see changes in root phenotypes of Arabidopsis. Our results for cell growth indicate that plant growth is highly influenced by the activity of the pumps by acidification of the apoplast causing wall loosening events in accordance with the Acid Growth Theory. This was shown by all four of the 101 overexpression lines LHA2-2C, LHA2-3C, LHA2-6H and LHA2-12K exhibiting greater primary root growth than the wild type in our root growth experiments on normal medium. There also is support for the H+ pumps to be involved with the initiation of lateral roots as 3 of the 4 overexpression lines have greater lateral root densities, suggesting that the activation of the pump by auxin begins movement of protons into the apoplast causing alkalinization events in the cytoplasm which may be the trigger involved with re-entry of the founder cells into the cell cycle. Separating the effects of auxin flow and auxin activation of the endogenous H+ pumps, we were able to reasonably ascertain that the H+ pump is involved with acid growth. Overexpression plants grown on the receptor based, auxin transport inhibitor, NPA revealed greater lateral root densities and primary root growth consistent with our hypothesis that an activated pump will retain primary root growth and increase lateral root numbers in transgenic plants compared to wild-type Arabidopsis. If NPA was able to dampen the effects of auxin flow and our hypotheses about acid growth and lateral root ontogeny are correct then this would explain why there were longer primary roots and greater lateral root densities of the overexpression lines over the wild-type lines because of increased activity of the pumps causing acid induced cell elongation and triggering lateral root initiation through cytoplasmic pH fluctuations. Our TIBA results with lateral roots helped to strengthen our ideas that the H+ pump can be involved as an early signaler in lateral root ontogeny. The TIBA primary root results, while not what we were anticipating, suggests that our data must be reconciled with further experimentation. Finally, our extracellular pH experiments provided a direct test to functionality of our H+ pumps in acidification and 102 supported a key element in our hypothesis that the H+ pumps were directly involved with acidification. This study helps support the Acid Growth Theory by providing a straightforward understanding of both the functionality and phenotypic changes surrounding the plant root with activated H+ pumps by utilizing an overexpression system. It also helps support the hypothesis that an integral signaling or mechanism involved in lateral root initiation is the H+ pumps and its ability to cause transient intracellular pH alkalinization. Future Directions This study characterized the roots of lines overexpressing a single copy of the plasma membrane H+-ATPase. We have successfully determined the presence of gene and RNA however we only have indirect tests for the protein through activity and phenotypic root evidence since we are missing a direct test for pump protein. A western blot using antibodies directed to our LHA2 transgene will provide direct evidence that the protein is indeed present. It would also be interesting to visualize, using fluorescent confocal or light microscopy, cytoplasmic pH or immunohistochemistry in the development of lateral roots to strengthen our idea that pH and the H+ pumps are directly involved in lateral root initiation. This study also touched upon germination effects that the overexpression exerts on the plant, we are currently exploring a phenotype where germination rates are decreased in overexpression lines compared to the wild-type (Figure 32). Germination is a highly ordered process and can be further studied to determine the effects of the H+ 103 pump on plant germination. Along those lines, are responses of overexpression of the H+ pump to environmental conditions such as high salt, heavy metals or drought conditions. Currently, experiments are underway looking at the H+ pump and salt, looking at different mechanisms that the H+ pump are involved, expression seems to be directed differently in the overexpression lines compared to the wild-type. 104 Literature Cited An, N.D., Wang, L. J., Ding, C. and XU, Z. H. (2001) Auxin distribution and transport during embryogenesis and seedgermination of Arabidopsis. Cell Research, 11, 273-278. Arango, M., Gevaudant, F., Oufattole, M., Boutry, M. (2003) The plasma membrane proton pump ATPase: the significance of gene subfamilies. Planta, 216, 355-365. Baekgaard, L., Fuglsang, A.T., Palmgren, M.G. (2005) Regulation of plant plasma membrane H+- and Ca2+-ATPases by terminal domains. Journal Bioenerg Biomembr, 37, 369-374. Beeckman, T., Burssens, S., Inze, D. (2001) The peri-cell-cycle in Arabidopsis. Journal of Experimental Botany, 52, 403-411. Benfey, P.N. and Chua, N.H. (1990) The Cauliflower Mosaic Virus 35S Promoter: Combinatorial Regulation of Transcription in Plants. Science, 250, 959-966. Berg, L.R. (2007) Introductory Botony: Plants, People and the Environment. Thompson Brooks/Cole, Belmont: 622 pp. Blakely, L.M., Durham, T.A. and Blakley R.M. (1982) Experimental Studies on Lateral Root Formation in Radish Seedling Roots. I. General Methods, Developmental Stages, and Spontaneous Formation of Laterals. Botanical Gazette, 143, 341-352. Blakeslee, J.J., Peer, W.A., Murphy, A.S. (2005) Auxin transport. Current Opinion in Plant Biology, 8, 494-500. Bonner, J. (1934) Studies on the Growth Hormone of Plants: V. The Relation of Cell Elongation to Cell Wall Formation. Proceedings of the National Academy of Sciences of the United States of America, 20, 393-397. Bradshaw, H.D., Jr. (2005) Mutations in CAX1 produce phenotypes characteristic of plants tolerant to serpentine soils. The New Phytologist, 167, 81-88. Bradshaw, K.G. (2001) The effect of an auxin transport inhibitor n-1-naphthylphthalamic acid (NPA) and sucrose on expression of LHA2 in Arabidopsis thaliana and Lycopersicon esculentum. Master's Thesis, California State University, Sacramento, Sacramento. Campbell, N.A. (1996) Biology. The Benjamin/Cummings Publishing company: 1206 pp. 105 Casimiro, I., Beeckman, T., Graham, N., Bhalerao, R., Zhang, H., Casero, P., Sandberg, G., Bennett, M.J. (2003) Dissecting Arabidopsis lateral root development. Trends in Plant Science, 8, 165-171. Casimiro, I., Marchant, A., Bhalerao, R.P., Beeckman, T., Dhooge, S., Swarup, R., Graham, N., Inze, D., Sandberg, G., Casero, P.J., Bennett, M. (2001) Auxin transport promotes Arabidopsis lateral root initiation. Plant Cell, 13, 843-852. Cheng, N.H., Pittman, J.K., Barkla, B.J., Shigaki, T., Hirschi, K.D. (2003) The Arabidopsis cax1 mutant exhibits impaired ion homeostasis, development, and hormonal responses and reveals interplay among vacuolar transporters. The Plant Cell, 15, 347-364. Cleland, R. (1971) The mechanical behavior of isolated Avena coleoptile walls subjected to constant stress: properties and relation to cell elongation. Plant Physiology, 47, 805-811. Cleland, R.E. (1992) Auxin-induced growth of Avena coleoptiles involves two mechanisms with different pH optima. Plant Physiology, 99, 1556-1561. Clough, S.J. and Bent, A.F. (1998) Floral dip: a simplified method for Agrobacteriummediated transformation of Arabidopsis thaliana. Plant Journal, 16, 735-743. Cosgrove, D.J. (2000) Loosening of plant cell walls by expansins. Nature, 407, 321-326. Cosgrove, D.J. (2005) Growth of the plant cell wall. National Revue Molecular Cell Biology, 6, 850-861. Dani, M. (2007) Regulation of the promoter of the tomato plasma membrane H⁺-ATPase, LHA2 by auxin in tomato (Solanum lycopersicum) and Arabidopsis thaliana. Masters Thesis, California State University, Sacramento, Sacramento. Darginaviciene, J., Jurkoniene, S., Bareikiene, N., and Šveikauskas, V. (2008) H+ATPase functional activity in plant cell plasma membrane. Scientific works of the Lithuanian Institute of Horticulture and Lithuanian University of Agriculture, 27 . Dubrovsky, J.G., Doerner, P.W., Colon-Carmona, A., Rost, T.L. (2000) Pericycle cell proliferation and lateral root initiation in Arabidopsis. Plant Physiology, 124, 1648-1657. Dubrovsky, J.G., Rost, T.L., Colon-Carmona, A., Doerner, P. (2001) Early primordium morphogenesis during lateral root initiation in Arabidopsis thaliana. Planta, 214, 30-36. 106 Duby, G. and Boutry, M. (2009) The plant plasma membrane proton pump ATPase: a highly regulated P-type ATPase with multiple physiological roles. Pflugers Arch, 457, 645-655. Duby, G., Poreba, W., Piotrowiak, D., Bobik, K., Derua, R., Waelkens, E., Boutry, M. (2009) Activation of plant plasma membrane H+-ATPase by 14-3-3 proteins is negatively controlled by two phosphorylation sites within the H+-ATPase Cterminal region. Journal Biological Chemistry, 284, 4213-4221. Ekberg, K., Palmgren, M.G., Veierskov, B., Buch-Pedersen, M.J. (2010) A novel mechanism of P-type ATPase autoinhibition involving both termini of the protein. The Journal of Biological chemistry, 285, 7344-7350. Estelle, M. (2001) Plant hormones. Transporters on the move. Nature, 413, 374-375. Ewing, N.N. and Bennett, A.B. (1994) Assessment of the number and expression of Ptype H(+)-ATPase genes in tomato. Plant Physiology, 106, 547-557. Ewing, N.N., Wimmers, L.E., Meyer, D.J., Chetelat, R.T., Bennett, A.B. (1990) Molecular Cloning of Tomato Plasma Membrane H-ATPase. Plant Physiol, 94, 1874-1881. Exposito-Rodriguez, M., Borges, A.A., Borges-Perez, A., Perez, J.A. (2008) Selection of internal control genes for quantitative real-time RT-PCR studies during tomato development process. BMC Plant Biology, 8, 131. Frias, I., Caldeira, M.T., Perez-Castineira, J.R., Navarro-Avino, J.P., Culianez-Macia, F.A., Kuppinger, O., Stransky, H., Pages, M., Hager, A., Serrano, R. (1996) A major isoform of the maize plasma membrane H(+)-ATPase: characterization and induction by auxin in coleoptiles. Plant Cell, 8, 1533-1544. Fukuda, A. and Tanaka, Y. (2006) Effects of ABA, auxin, and gibberellin on the expression of genes for vacuolar H+ -inorganic pyrophosphatase, H+ -ATPase subunit A, and Na+/H+ antiporter in barley. Plant physiology and biochemistry : PPB / Societe Francaise de Physiologie Vegetale, 44, 351-358. Gambetta, G. (2005) Characterization of the Expression Pattern of the Tomato H+ ATPase LHA2 Promoter During Plant Development. Masters Thesis. California State University Sacramento, Sacramento. Gaxiola, R.A., Li, J., Undurraga, S., Dang, L.M., Allen, G.J., Alper, S.L., Fink, G.R. (2001) Drought- and salt-tolerant plants result from overexpression of the AVP1 H+-pump. Proc Natl Acad Sci U S A, 98, 11444-11449. 107 Gaxiola, R.A., Palmgren, M.G., Schumacher, K. (2007) Plant proton pumps. FEBS Lett, 581, 2204-2214. Gevaudant, F., Duby, G., von Stedingk, E., Zhao, R., Morsomme, P., Boutry, M. (2007) Expression of a constitutively activated plasma membrane H+-ATPase alters plant development and increases salt tolerance. Plant Physiol, 144, 1763-1776. Gil, P., Liu, Y., Orbovic, V., Verkamp, E., Poff, K.L., Green, P.J. (1994) Characterization of the auxin-inducible SAUR-AC1 gene for use as a molecular genetic tool in Arabidopsis. Plant Physiology, 104, 777-784. Guilfoyle, T.J. and Hagen, G. (2007) Auxin response factors. Current Opinion Plant Biology, 10, 453-460. Hagen, G. and Guilfoyle, T. (2002) Auxin-responsive gene expression: genes, promoters and regulatory factors. Plant Molecular Biology, 49, 373-385. Hager, A. (2003) Role of the plasma membrane H+-ATPase in auxin-induced elongation growth: historical and new aspects. Journal of Plant Research, 116, 483-505. Hager, A., Debus, G., Edel, H.G., Stransky, H., Serrano, R. (1991) Auxin Induces Exocytosis and the Rapid Synthesis of a High-Turnover Pool of Plasmamembrane H+ -ATPase. Planta, 185, 527-537. Hager, A., Menzel, H., and Krauss, A. (1971) Versuche und Hypothese zur Primarwirkung des Auxins beim Streckungswachstum. Planta, 100, 47-75. Harguindey, S., Orive, G., Luis Pedraz, J., Paradiso, A., Reshkin, S.J. (2005) The role of pH dynamics and the Na+/H+ antiporter in the etiopathogenesis and treatment of cancer. Two faces of the same coin--one single nature. Biochim Biophys Acta, 1756, 1-24. Harmon, B. and Sedat, J. (2005) Cell-by-cell dissection of gene expression and chromosomal interactions reveals consequences of nuclear reorganization. Public Library of Science Biology, 3, e67. Haruta, M., Burch, H.L., Nelson, R.B., Barrett-Wilt, G., Kline, K.G., Mohsin, S.B., Young, J.C., Otegui, M.S., Sussman, M.R. (2010) Molecular characterization of mutant Arabidopsis plants with reduced plasma membrane proton pump activity. The Journal of Biological Chemistry, 285, 17918-17929. Himanen, K., Boucheron, E., Vanneste, S., de Almeida Engler, J., Inze, D., Beeckman, T. (2002) Auxin-mediated cell cycle activation during early lateral root initiation. Plant Cell, 14, 2339-2351. 108 Idate, R. (1997) Identification and characterization of the promoter region of the tomato plasma membrane proton atpase gene LHA2. Masters Thesis, California State University, Sacramento, Sacramento. Kalampanayil, B.D., and Wimmers, L.E. (2001) Identification and characterization of a salt-stree-induced plasma membrane H+-ATPase in tomato. Plant, Cell and Environment, 24, 999-1005. Kotake, T., Nakagawa, N., Takeda, K., Sakurai, N. (2000) Auxin-induced elongation growth and expressions of cell wall-bound exo- and endo-beta-glucanases in barley coleoptiles. Plant Cell Physiology, 41, 1272-1278. Kutschera, U. and Niklas, K.J. (2007) The epidermal-growth-control theory of stem elongation: an old and a new perspective. Journal of Plant Physiology, 164, 13951409. Kutschera, U.a.S., P. (1985) Evidence against the acid-growth theory of auxin action. Planta, 483-493. Lee, J. (2007) The Effect of Overexpression of Tomato H+ Atapase isoform LHA2 on the Growth and Development of Arabidopsis thaliana. Masters Thesis. California State University, Sacramento, Sacramento. Li, J., Yang, H., Peer, W.A., Richter, G., Blakeslee, J., Bandyopadhyay, A., Titapiwantakun, B., Undurraga, S., Khodakovskaya, M., Richards, E.L., Krizek, B., Murphy, A.S., Gilroy, S., Gaxiola, R. (2005) Arabidopsis H+-PPase AVP1 regulates auxin-mediated organ development. Science, 310, 121-125. Li, L. and Steffens, J.C. (2002) Overexpression of polyphenol oxidase in transgenic tomato plants results in enhanced bacterial disease resistance. Planta, 215, 239247. Malamy, J.E. and Ryan, K.S. (2001) Environmental regulation of lateral root initiation in Arabidopsis. Plant Physiol, 127, 899-909. Maurel, C., Leblanc, N., Barbier-Brygoo, H., Perrot-Rechenmann, C., Bouvier-Durand, M., Guern, J. (1994) Alterations of auxin perception in rolB-transformed tobacco protoplasts. Time course of rolB mRNA expression and increase in auxin sensitivity reveal multiple control by auxin. Plant Physiology, 105, 1209-1215. McQueen-Mason, S.J. and Cosgrove, D.J. (1995) Expansin mode of action on cell walls. Analysis of wall hydrolysis, stress relaxation, and binding. Plant Physiology, 107, 87-100. 109 Mills, R.F., Krijger, G.C., Baccarini, P.J., Hall, J.L., Williams, L.E. (2003) Functional expression of AtHMA4, a P1B-type ATPase of the Zn/Co/Cd/Pb subclass. The Plant Journal: for Cell and Molecular Biology, 35, 164-176. Mockaitis, K. and Estelle, M. (2008) Auxin receptors and plant development: a new signaling paradigm. Annual Revue Cell Developmental Biology, 24, 55-80. Murphy, A., Peer, W.A., Taiz, L. (2000) Regulation of auxin transport by aminopeptidases and endogenous flavonoids. Planta, 211, 315-324. Nishiyama, H., Ohya, T., Tanoi, K., and Nakanishi, T. M. (2007) A simple measurement of the pH of root apoplast by the flourecence ratio method. Plant Root, 3-6. Palmgren, M.G. (1990) An H-ATPase Assay: Proton Pumping and ATPase Activity Determined Simultaneously in the Same Sample. Plant Physiology, 94, 882-886. Palmgren, M.G. (2001) PLANT PLASMA MEMBRANE H+-ATPases: Powerhouses for Nutrient Uptake. Annual Revue Plant Physiology Plant Molecular Biology, 52, 817-845. Pardo, J.M. and Serrano, R. (1989) Structure of a plasma membrane H+-ATPase gene from the plant Arabidopsis thaliana. Journal of Biological Chemistry, 264, 85578562. Pasternak, T.P., Prinsen, E., Ayaydin, F., Miskolczi, P., Potters, G., Asard, H., Van Onckelen, H.A., Dudits, D., Feher, A. (2002) The Role of auxin, pH, and stress in the activation of embryogenic cell division in leaf protoplast-derived cells of alfalfa. Plant Physiology, 129, 1807-1819. Pedersen, B.P., Buch-Pedersen, M.J., Morth, J.P., Palmgren, M.G., Nissen, P. (2007) Crystal structure of the plasma membrane proton pump. Nature, 450, 1111-1114. Peer, W.A., Bandyopadhyay, A., Blakeslee, J.J., Makam, S.N., Chen, R.J., Masson, P.H., Murphy, A.S. (2004) Variation in expression and protein localization of the PIN family of auxin efflux facilitator proteins in flavonoid mutants with altered auxin transport in Arabidopsis thaliana. The Plant Cell, 16, 1898-1911. Perona, R., Portillo, F., Giraldez, F., Serrano, R. (1990) Transformation and pH homeostasis of fibroblasts expressing yeast H(+)-ATPase containing site-directed mutations. Molecular Cellular Biology, 10, 4110-4115. Perona, R. and Serrano, R. (1988) Increased pH and tumorigenicity of fibroblasts expressing a yeast proton pump. Nature, 334(6181), 438-440. 110 Pichon, O.a.D., M. (1994) Is cytoplasmic pH involved in the regulation of cell cycle in plants? Physiologica Plantarum (92), 261-265. Pitann, B., Kranz, T., and Muhling, K. (2009) The apoplastic pH and its significance in adaption to salinity in maize (Zea mays L.): Comparison of fluorescence microscopy and ph-sensitive microelectrodes. Plant Science, 176, 497-504. Portillo, F. (2000) Regulation of plasma membrane H(+)-ATPase in fungi and plants. Biochim Biophys Acta, 1469, 31-42. Rahman, A., Bannigan, A., Sulaman, W., Pechter, P., Blancaflor, E.B., Baskin, T.I. (2007) Auxin, actin and growth of the Arabidopsis thaliana primary root. Plant Journal, 50, 514-528. Rayle, D.L. and Cleland, R. (1970) Enhancement of wall loosening and elongation by Acid solutions. Plant Physiology, 46, 250-253. Rayle, D.L. and Cleland, R.E. (1992a) The Acid Growth Theory of auxin-induced cell elongation is alive and well. Plant Physiology, 99, 1271-1274. Regenberg, B., Villalba, J.M., Lanfermeijer, F.C., Palmgren, M.G. (1995) C-terminal deletion analysis of plant plasma membrane H(+)-ATPase: yeast as a model system for solute transport across the plant plasma membrane. Plant Cell, 7, 16551666. Ro, S. (2000) Transcriptional regulation of genes controlling plant growth and development: LHA2, a H+-ATPase, and LeEXP1, and expansin. Masters Thesis. California State University, Sacramento, Sacramento. Rober-Kleber, N., Albrechtova, J.T., Fleig, S., Huck, N., Michalke, W., Wagner, E., Speth, V., Neuhaus, G., Fischer-Iglesias, C. (2003) Plasma membrane H+-ATPase is involved in auxin-mediated cell elongation during wheat embryo development. Plant Physiol, 131, 1302-1312. Robertson, W.R., Clark, K., Young, J.C., Sussman, M.R. (2004) An Arabidopsis thaliana plasma membrane proton pump is essential for pollen development. Genetics, 168, 1677-1687. Schmittgen, T.D. and Zakrajsek, B.A. (2000) Effect of experimental treatment on housekeeping gene expression: validation by real-time, quantitative RT-PCR. Journal of Biochemical and Biophysical Methods, 46, 69-81. Stals, H. and Inze, D. (2001) When plant cells decide to divide. Trends in Plant Science, 6, 359-364. 111 Staswick, P.E., Serban, B., Rowe, M., Tiryaki, I., Maldonado, M.T., Maldonado, M.C., Suza, W. (2005) Characterization of an Arabidopsis enzyme family that conjugates amino acids to indole-3-acetic acid. Plant Cell, 17, 616-627. Taiz, L. and Zeiger, E. (2010) Plant Physiology. Sinauer Associates, Sunderland, MA. 782 pp. Taylor, C.B. (1997) Comprehending Cosuppression. The Plant Cell, 9(1245). Tiwari, S.B., Hagen, G., Guilfoyle, T. (2003) The roles of auxin response factor domains in auxin-responsive transcription. Plant Cell, 15, 533-543. Turner, T.L., Bourne, E.C., Von Wettberg, E.J., Hu, T.T., Nuzhdin, S.V. (2010) Population resequencing reveals local adaptation of Arabidopsis lyrata to serpentine soils. Nature Genetics, 42, 260-263. Woodward, A.W. and Bartel, B. (2005) Auxin: regulation, action, and interaction. Annual Botanical (London), 95, 707-735. Wu, X.a.M., P. (2007) The role of auxin tansport during inflorescence development in Maize (Zea mays, Poaceae). American Journal of Botany, 94, 1745-1755. Yang, X., Feng, Y., He, Z., Stoffella, P.J. (2005) Molecular mechanisms of heavy metal hyperaccumulation and phytoremediation. Journal of Trace Elements in Medicine and Biology: Organ of the Society for Minerals and Trace Elements, 18, 339-353. Yu, Q., Kuo, J., and Tang, C. (2001) Using confocal laser scanning microscopy to measure apoplastic pH change in roots of Lupinus angustifolius L. in response to high pH. Annals of Botany, 87, 47-52. Zhao, R., Dielen, V., Kinet, J.M., Boutry, M. (2000) Cosuppression of a plasma membrane H(+)-ATPase isoform impairs sucrose translocation, stomatal opening, plant growth, and male fertility. The Plant Cell, 12, 535-546.