REDCap - Indiana University
advertisement

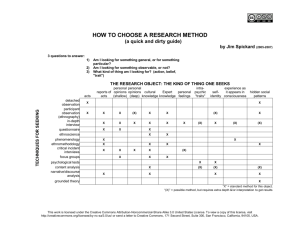
Easy creation of data management systems, plus a lot more Andy Arenson, Advanced Biomedical IT Core Bob Davis, Biostatistics © Trustees of Indiana University Released under Creative Commons 3.0 unported license; license terms on last slide. ACCELERATING CLINICAL AND TRANSLATIONAL RESEARCH www.indianactsi.org Agenda • Review of REDCap basics • Advanced topics • When REDCap alone isn’t enough © Trustees of Indiana University Released under Creative Commons 3.0 unported license; license terms on last slide. ACCELERATING CLINICAL AND TRANSLATIONAL RESEARCH www.indianactsi.org What is REDCap? • Easily create a web-based data management system – PHP, Javascript, MySQL • Originally for clinical research, but usable in other domains • Developed by Vanderbilt. – 460 institutional partners (as of 10-Sep-2012) – http://www.project-redcap.org © Trustees of Indiana University Released under Creative Commons 3.0 unported license; license terms on last slide. ACCELERATING CLINICAL AND TRANSLATIONAL RESEARCH www.indianactsi.org REDCap Consortium © Trustees of Indiana University Released under Creative Commons 3.0 unported license; license terms on last slide. • Worldwide: 45,863 studies; 61,435 end-users ACCELERATING CLINICAL AND TRANSLATIONAL RESEARCH www.indianactsi.org REDCap Advantages • No programming • Easy to create / Get started fast • Version Control – Always know where the latest data is • Data Consistency – Always spell things the same way • Accessible from web (use IU, PU, ND passphrase) • Logging – What changes were made by whom © Trustees of Indiana University Released under Creative Commons 3.0 unported license; license terms on last slide. ACCELERATING CLINICAL AND TRANSLATIONAL RESEARCH www.indianactsi.org Demonstration: Start to Finish https://redcap.uits.iu.edu • Create a database • Data Entry • Data Export © Trustees of Indiana University Released under Creative Commons 3.0 unported license; license terms on last slide. ACCELERATING CLINICAL AND TRANSLATIONAL RESEARCH www.indianactsi.org What else can REDCap do? • Analysis/Management – Report Builder – Graphical Data View & Stats – Deidentification • Quality Assurance – Double Data Entry – Data Quality • Other Types of Data – Surveys – Longitudinal Data • Operations – Calendar & Scheduling – Data Access Groups © Trustees of Indiana University Released under Creative Commons 3.0 unported license; license terms on last slide. ACCELERATING CLINICAL AND TRANSLATIONAL RESEARCH www.indianactsi.org Report Builder © Trustees of Indiana University Released under Creative Commons 3.0 unported license; license terms on last slide. ACCELERATING CLINICAL AND TRANSLATIONAL RESEARCH www.indianactsi.org Graphical Data View & Stats © Trustees of Indiana University Released under Creative Commons 3.0 unported license; license terms on last slide. ACCELERATING CLINICAL AND TRANSLATIONAL RESEARCH www.indianactsi.org Deidentification © Trustees of Indiana University Released under Creative Commons 3.0 unported license; license terms on last slide. ACCELERATING CLINICAL AND TRANSLATIONAL RESEARCH www.indianactsi.org Deidentification 2 © Trustees of Indiana University Released under Creative Commons 3.0 unported license; license terms on last slide. ACCELERATING CLINICAL AND TRANSLATIONAL RESEARCH www.indianactsi.org Quality Assurance • Double Data Entry – Compare data entry by two different people • Data Quality – Use rules to check for discrepancies in the data © Trustees of Indiana University Released under Creative Commons 3.0 unported license; license terms on last slide. ACCELERATING CLINICAL AND TRANSLATIONAL RESEARCH www.indianactsi.org Double Data Entry © Trustees of Indiana University Released under Creative Commons 3.0 unported license; license terms on last slide. ACCELERATING CLINICAL AND TRANSLATIONAL RESEARCH www.indianactsi.org Data Quality © Trustees of Indiana University Released under Creative Commons 3.0 unported license; license terms on last slide. ACCELERATING CLINICAL AND TRANSLATIONAL RESEARCH www.indianactsi.org Data Quality 2 © Trustees of Indiana University Released under Creative Commons 3.0 unported license; license terms on last slide. ACCELERATING CLINICAL AND TRANSLATIONAL RESEARCH www.indianactsi.org Other Types of Data • Surveys – Participants enter their own data • Longitudinal Data – Forms can be reused for multiple Events © Trustees of Indiana University Released under Creative Commons 3.0 unported license; license terms on last slide. ACCELERATING CLINICAL AND TRANSLATIONAL RESEARCH www.indianactsi.org Surveys © Trustees of Indiana University Released under Creative Commons 3.0 unported license; license terms on last slide. ACCELERATING CLINICAL AND TRANSLATIONAL RESEARCH www.indianactsi.org Longitudinal Data © Trustees of Indiana University Released under Creative Commons 3.0 unported license; license terms on last slide. ACCELERATING CLINICAL AND TRANSLATIONAL RESEARCH www.indianactsi.org Operations • Data Access Groups – Allow people at multiple sites to contribute to the same database, without seeing other people’s data. • Calendar & Scheduling – Create a list of upcoming events and track the status of those events. © Trustees of Indiana University Released under Creative Commons 3.0 unported license; license terms on last slide. ACCELERATING CLINICAL AND TRANSLATIONAL RESEARCH www.indianactsi.org Data Access Groups © Trustees of Indiana University Released under Creative Commons 3.0 unported license; license terms on last slide. ACCELERATING CLINICAL AND TRANSLATIONAL RESEARCH www.indianactsi.org Calendar & Scheduling © Trustees of Indiana University Released under Creative Commons 3.0 unported license; license terms on last slide. ACCELERATING CLINICAL AND TRANSLATIONAL RESEARCH www.indianactsi.org REDCap as a Platform for more • Interoperate with other programs via Application Programmer Interface (API) – Web Services interface for reading and writing data – Example: Data Transfer Service – incorporates data from other database, such as lab results. • Add new REDCap functionality with Plugins – PHP code on same server as REDCap – Can not replace existing functionality © Trustees of Indiana University Released under Creative Commons 3.0 unported license; license terms on last slide. ACCELERATING CLINICAL AND TRANSLATIONAL RESEARCH www.indianactsi.org REDCap: What does it cost? Do-it-yourself Fee for Service Basic & Advanced Functionality API-based programs Plugins © Trustees of Indiana University Released under Creative Commons 3.0 unported license; license terms on last slide. ACCELERATING CLINICAL AND TRANSLATIONAL RESEARCH www.indianactsi.org Example of going beyond the obvious • REDCap Survey used to help step through a flowchart used to provide implant guidelines – https://redcap.uits.iu.edu/surveys/?s=L4XCe7 © Trustees of Indiana University Released under Creative Commons 3.0 unported license; license terms on last slide. ACCELERATING CLINICAL AND TRANSLATIONAL RESEARCH www.indianactsi.org Questions? hubsupport@indianactsi.org © Trustees of Indiana University Released under Creative Commons 3.0 unported license; license terms on last slide. ACCELERATING CLINICAL AND TRANSLATIONAL RESEARCH www.indianactsi.org Acknowledgements • Funding for the IndianaCTSI instance of REDCap comes in part from the NIH Clinical & Translational Science Awards (http://www.ncats.nih.gov/research/cts/ctsa/ctsa.html). © Trustees of Indiana University Released under Creative Commons 3.0 unported license; license terms on last slide. ACCELERATING CLINICAL AND TRANSLATIONAL RESEARCH www.indianactsi.org Appendices • • • • • Where is REDCap? Who already uses REDCap? What data can REDCap manage? What data is not allowed in REDCap? Who can use REDCap • Screenshots of Basic functionality © Trustees of Indiana University Released under Creative Commons 3.0 unported license; license terms on last slide. ACCELERATING CLINICAL AND TRANSLATIONAL RESEARCH www.indianactsi.org Where is REDCap? • Hosted on a physical server in the Bloomington Data Center • Supported by the Indiana Clinical Translational Sciences Institute (CTSI) via a collaboration of staff from the Biostatistics Department and from the Advanced IT Core of the IU School of Medicine, which is part of Research Technologies/UITS © Trustees of Indiana University Released under Creative Commons 3.0 unported license; license terms on last slide. ACCELERATING CLINICAL AND TRANSLATIONAL RESEARCH www.indianactsi.org Who already uses REDCap? • Statistics (as of 10-Sep-2012): – 795 dbs (380 production, 346 development, 14 inactive, 55 archived) – 641 active users – 2,657 data entry forms; 79,664 data fields; 233,163 data records • Who – – – – IU School of Medicine – many departments, divisions/sections IU School of Nursing, IU School of Dentistry, Indiana CTSI Regenstrief Institute, Purdue University, University of Notre Dame IU Health – Methodist; Wishard Health Services; Rehabilitation Hospital of Indiana © Trustees of Indiana University Released under Creative Commons 3.0 unported license; license terms on last slide. ACCELERATING CLINICAL AND TRANSLATIONAL RESEARCH www.indianactsi.org What data can REDCap manage? • Numbers, text, dates, files, lists (radio/checkbox/multiple), branching, calculations • Simple Schemas – Designed for a single table of information – 1:many relationship is possible, but tedious to create – Many:many only possible by using multiple REDCap databases. • Electronic Protected Health Information (ePHI) – IU has put in place appropriate administrative, technical, and © Trustees of Indiana University physical controls to protect data in accordance with the HIPAA Released under Creative Commons 3.0 unported license; license terms on last slide. security rule ACCELERATING CLINICAL AND TRANSLATIONAL RESEARCH www.indianactsi.org What data is not allowed in REDCap? • When storing ePHI, you remain responsible for privacy, security, and compliance. You need IRB approval and a Data Management Plan. Technical and physical controls are provided, but you remain responsible for administrative controls. • REDCap is for use only in support of academic research. Do NOT use REDCap for other uses, for instance to store official university business data, patient care data, or electronic health records (EHRs). © Trustees of Indiana University Released under Creative Commons 3.0 unported license; license terms on last slide. ACCELERATING CLINICAL AND TRANSLATIONAL RESEARCH www.indianactsi.org Who can use REDCap? • Anyone at an Indiana CTSI member organization (IU, Purdue, Notre Dame) plus any of their partners at other institutions. • Register an IndianaCTSI HUB account: https://www.indianactsi.org/register • Request a REDCap account: http://www.indianactsi.org/redcapacr© Trustees of Indiana University Released under Creative Commons 3.0 unported license; license terms on last slide. ACCELERATING CLINICAL AND TRANSLATIONAL RESEARCH www.indianactsi.org Data Dictionary - GUI © Trustees of Indiana University Released under Creative Commons 3.0 unported license; license terms on last slide. ACCELERATING CLINICAL AND TRANSLATIONAL RESEARCH www.indianactsi.org Data Dictionary - GUI © Trustees of Indiana University Released under Creative Commons 3.0 unported license; license terms on last slide. ACCELERATING CLINICAL AND TRANSLATIONAL RESEARCH www.indianactsi.org Data Dictionary – Resulting Form © Trustees of Indiana University Released under Creative Commons 3.0 unported license; license terms on last slide. ACCELERATING CLINICAL AND TRANSLATIONAL RESEARCH www.indianactsi.org Data Export © Trustees of Indiana University Released under Creative Commons 3.0 unported license; license terms on last slide. ACCELERATING CLINICAL AND TRANSLATIONAL RESEARCH www.indianactsi.org Data Dictionary – Spreadsheet © Trustees of Indiana University Released under Creative Commons 3.0 unported license; license terms on last slide. ACCELERATING CLINICAL AND TRANSLATIONAL RESEARCH www.indianactsi.org Data Dictionary – Spreadsheet © Trustees of Indiana University Released under Creative Commons 3.0 unported license; license terms on last slide. ACCELERATING CLINICAL AND TRANSLATIONAL RESEARCH www.indianactsi.org Data Dictionary – Spreadsheet Upload © Trustees of Indiana University Released under Creative Commons 3.0 unported license; license terms on last slide. ACCELERATING CLINICAL AND TRANSLATIONAL RESEARCH www.indianactsi.org Data Dictionary – Library © Trustees of Indiana University Released under Creative Commons 3.0 unported license; license terms on last slide. ACCELERATING CLINICAL AND TRANSLATIONAL RESEARCH www.indianactsi.org Audit trails & Logging © Trustees of Indiana University Released under Creative Commons 3.0 unported license; license terms on last slide. ACCELERATING CLINICAL AND TRANSLATIONAL RESEARCH www.indianactsi.org Acknowledgements & disclaimer • • • This material is based upon work supported by the NIH under the Clinical & Translational Science Awards (http://www.ncats.nih.gov/research/cts/ctsa/ctsa.html) This work was supported in part by the Lilly Endowment, Inc. and the Indiana University Pervasive Technology Institute Any opinions presented here are those of the presenter(s) and do not necessarily represent the opinions of the National Institutes of Health or any other funding agencies ACCELERATING CLINICAL AND TRANSLATIONAL RESEARCH www.indianactsi.org License terms • Please cite as: Arenson, A. D. REDCap: Easy creation of data management systems, plus a lot more. (Presentation) Conference (Indiana University Statewide IT Conference, 24 September 2012). Available from: http://hdl.handle.net/2022/14738 • Items indicated with a © are under copyright and used here with permission. Such items may not be reused without permission from the holder of copyright except where license terms noted on a slide permit reuse. • Except where otherwise noted, contents of this presentation are copyright 2012 by the Trustees of Indiana University. • This document is released under the Creative Commons Attribution 3.0 Unported license (http://creativecommons.org/licenses/by/3.0/). This license includes the following terms: You are free to share – to copy, distribute and transmit the work and to remix – to adapt the work under the following conditions: attribution – you must attribute the work in the manner specified by the author or licensor (but not in any way that suggests that they endorse you or your use of the work). For any reuse or distribution, you must make clear to others the license terms of this work. ACCELERATING CLINICAL AND TRANSLATIONAL RESEARCH www.indianactsi.org