Field Methods of Monitoring Atmospheric Systems
advertisement
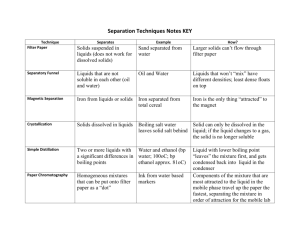
Field Methods of Monitoring Aquatic Systems Unit 3 – Suspended and Dissolved Solids Copyright © 2006 by DBS Title Total dissolved solids is a good measure of the concentration of ionic substances in water Solids • • • • • • Solids are categorized into several groups based on particle size and characterization – Total Suspended Solids (TSS) – Total Dissolved Solids (TDS) – Volatile Suspended Solids (VSS) – Total Solids (TS) Consist of an inorganic fraction (silts, clays, etc.) and an organic fraction (algae, zooplankton, bacteria, and detritus) Inorganic portion >>> organic Contribute to turbidity of the water High sediment loads are obvious because of their "muddy" appearance Drinking water limit 500 mg L-1 (secondary standard) Total Suspended SolidsCategories TSS are the amount of filterable solids in a water sample. Samples are filtered through a glass fiber filter. The filters are dried and weighed to determine total suspended solids in mg L-1 Total Dissolved Solids TDS are those solids (inorganic+organic) that pass through a filter of 2.0 μm or smaller. They are said to be non-filterable. After filtration the filtrate (liquid) is dried and the remaining residue is weighed and calculated as mg/l of Total Dissolved Solids Volatile Suspended Solids Volatile solids are those solids lost on ignition (heating to 550 °C.) They give a rough approximation of the amount of organic matter present in the solid fraction Total Solids TS are the total of all solids in a water sample. Can be measured by evaporation - problems with water retention and decomposition of OM TS = TSS + TDS + VSS Question What physical problems may be caused by suspended particles? Cut down light transmission and lower the rate of photosynthesis Clog fish gills In less turbulent parts of rivers and particularly in lakes particles sediment out (siltation) smothering the bottom dwellers Also affect temperature and DO (heat capacity) Sources of Error • • • • • Sampling, subsampling, pipeting 3-phase smples Keep homogeneous during transfer – do not pipet from vortex! Mix small samples with magnetic sampler Pipet SS with wide-bore pipet Analyze at least 10% samples in duplicate (should agree ± 5% average weight) • Dry to constant weight (or change is < 4% previous or 0.5 mg) • Record variations in procedures Sampling • Sample size needed will vary depending on turbidity – Choose sample volume to yield 2.5 - 200 mg dried residue – If longer than 10 min for filtration increase filter size or decrease sample volume • Store in the dark at 4 °C to minimize MO decomposition (for up to 7 days) • Analyze ASAP due to impracticality of preserving sample (preferably no longer than 24 h) • Bring to room temperature prior to analysis Analysis for TSS • Analysis via filtration and weighing – Hartley funnel or magnetic funnel – Glass fiber disc with membrane filter Hartley Funnel Magnetic Funnel Analysis for TSS Remove sample from refrigerator ~30 mins prior to analysis 1. 2. 3. 4. 5. 6. 7. 8. 9. Prepare a 0.45 micron filter using wash-dry-cool cycle Seat filter with DI water prior to filtration Pipet a measured volume into filtration apparatus from stirred sample Wash filter with 3 x 10 mL washings DI water Apply suction for 3 mins after washing Transfer to aluminum dish for support Dry in the oven at 103-105 °C overnight (should be 1 h) Place in a dessicator to cool to room temperature, reweigh to the nearest 0.1 mg Calculate TSS in mg L-1 Analysis for TDS Remove sample from refrigerator ~30 mins prior to analysis 1. Dry a clean dry beaker at 103-105 °C for at least 1 hour 2. Dessicate until cool and weigh to nearest 0.1 mg 3. Filter 50.0 mL through a 0.45 μm filter paper into clean dry beaker using technique described above 4. Heat the sample to just below boiling and reduce the volume to 10 mL DO NOT ALLOW TO BOIL OVER OR SPLATTER 5. Allow the beaker to cool and dry in the oven at 103-105 °C overnight 6. Remove the beaker and place in a dessicator to cool to room temperature, reweigh to the nearest 0.1 mg 7. Calculate TDS in mg L-1 Question Why is it necessary to wash filter with 3 x 10 mL washings of DI water? If water contains an appreciable amount of dissolved substances these will add to the weight of the filter as it is dried TDS Meter • Conductivity meter ‘in disguise’ • Only detects ions • Do not detect neutral species – Organics – Marcoscopic particles • Not really TDS • +/- 10% accuracy compared to TDS Turbidity • Turbidity - cloudiness or haziness of water caused by individual particles that are too small to be seen without magnification • In water quality monitoring situations, a series of more labor intensive TSS measurements will be paired with measurements from a nephalometer to develop a site-specific correlation Text Books • • • • • • • • Rump, H.H. (2000) Laboratory Manual for the Examination of Water, Waste Water and Soil. Wiley-VCH. Nollet, L.M. and Nollet, M.L. (2000) Handbook of Water Analysis. Marcel Dekker. Keith, L.H. and Keith, K.H. (1996) Compilation of Epa's Sampling and Analysis Methods. CRC Press. Van der Leeden, F., Troise, F.L., and Todd, D.K. (1991) The Water Encyclopedia. Lewis Publishers. Kegley, S.E. and Andrews, J. (1998) The Chemistry of Water. University Science Books. Narayanan, P. (2003) Analysis of environmental pollutants : principles and quantitative methods. Taylor & Francis. Reeve, R.N. (2002) Introduction to environmental analysis. Wiley. Clesceri, L.S., Greenberg, A.E., and Eaton, A.D., eds. (1998) Standard Methods for the Examination of Water and Wastewater, 20th Edition. Published by American Public Health Association, American Water Works Association and Water Environment Federation.