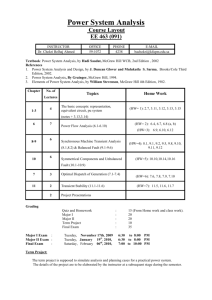
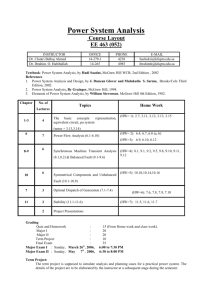
Chap 5-6: Seawater
advertisement

Chap 5-6: Seawater SPECIAL DATES: MPA meeting…6 Jul R/V Pt Sur Cruise…14 Jul R/V Pt Sur Cruise…25 Jul Exam-1 (definite)...2 Aug Exam-2 (Tentative)…1 Sep Labor Day Holiday...5 Sep Final Exam...19 Sep (Sp-226, 1300-1450) OC3230-Paduan OC3230 Calendar, Summer 2005 version 13 July 2005 EX 1 images Copyright © McGraw Hill Chap 5-6: Seawater (+) Water molecules are unique: 105˚ angle produces polar molecule act as tiny magnets-good dissolver Hydrogen bonds change the energy requirements for temperature and phase changes (-) OC3230-Paduan images Copyright © McGraw Hill Chap 5-6: Seawater OC3230-Paduan images Copyright © McGraw Hill Chap 5-6: Seawater OC3230-Paduan images Copyright © McGraw Hill Chap 5-6: Seawater 680 cal Water exists in all three phases at the Earth’s surface temperature range; Energy is required to change phase Units: 1 cal = 4.18 Joule OC3230-Paduan images Copyright © McGraw Hill Chap 5-6: Seawater Water has a very high heat capacity compared with most other substances OC3230-Paduan images Copyright © McGraw Hill Chap 5-6: Seawater Note variations for water in different phases OC3230-Paduan images Copyright © McGraw Hill Chap 5-6: Seawater Fresh water is unique in that its maximum density is not at the coldest temperature Also, the phase change from water to ice leads to an unusual decrease in -30 0.9200 -60 0.9224 -100 0.9257 density due to change in molecular packing 4˚ C water has max density(ice has a more implications for freshwater lakes and regular but less dense winter-time ecosystems tetrahedral structure) Ice floats!-also important for ecosystems look ahead: freezing problem OC3230-Paduan images Copyright © McGraw Hill Chap 5-6: Seawater Again, ice floats and 4˚ C water sinks (previous density versus temperature figure is more useful/important) OC3230-Paduan images Copyright © McGraw Hill Chap 5-6: Seawater What about salt water? Dissolving ability of water: molecules can surround and isolate charged ions as a function of the polar nature of the molecule Seawater has nearly all known elements dissolved within it OC3230-Paduan images Copyright © McGraw Hill Chap 5-6: Seawater Salinity: “The total amount of solid materials in grams contained in one kilogram of seawater when all the carbonate has been converted to oxide, the bromine and iodine replaced by chlorine, and all organic matter completely oxidized.” This is an operational definition related to the practical methods used to compute salinity Units: g/kg; parts per thousand, ppt (‰) Open Ocean Range: 34.0–35.5‰ Open Ocean Average: 35‰ Measurement Methods: 1) Evaporation, 2) Absolute Salinity, SA, from Chlorinity, and 3) Practical Salinity from conductivity OC3230-Paduan images Copyright © McGraw Hill Chap 5-6: Seawater Why are the oceans salty? 1) Rivers dump sediments into the oceans No evidence that salinity has been increasing over time but, rather, it has been about the same as it is now Composition of dissolved salts is not like typical river sediments 2) Mid-ocean ridges provide dissolved material to the oceans Composition of dissolved salts is more like material in ocean crust than river sediments Material from ridges are more soluble Chemical equilibrium keeps salinity constant OC3230-Paduan images Copyright © McGraw Hill Chap 5-6: Seawater A few major ions account for most of the dissolved material The six most common are: Cl-, Na+, SO42-, Mg2+, Ca2+, K+ Two diagrams illustrating fraction of major ions dissolved in seawater (nearly all other elements are also contained in trace amounts): ? OC3230-Paduan images Copyright © McGraw Hill Chap 5-6: Seawater Major ions in seawater are always found in the same ratio one to another This is known as the “Law of Constant Proportions” SA (‰) = 1.80655 x Cl OC3230-Paduan images Copyright © McGraw Hill Chap 5-6: Seawater OC3230-Paduan images Copyright © McGraw Hill Chap 5-6: Seawater Plants in the ocean require nutrients for cell growth and other chemosynthetic activities Major nutrients are present in the form of Nitrate (NO3-), Phosphate (PO43-), and Silicate (SiO44-) ions Availability of these nutrients can control productivity (e.g., upwelling supplies nutrients and supports growth) OC3230-Paduan images Copyright © McGraw Hill Chap 5-6: Seawater Plants in the ocean require nutrients for cell growth and other chemosynthetic activities ? Some trace elements, in particular iron (Fe), are now believed to also be necessary for growth OC3230-Paduan images Copyright © McGraw Hill Chap 5-6: Seawater Back to density of water… What happens when you add salt? Properties of the liquid that change when the concentration of dissolved materials changes are known as “Colligative Properties” Examples: Freezing point depression, boiling point elevation, and changes in the temperature of maximum density OC3230-Paduan images Copyright © McGraw Hill Chap 5-6: Seawater At typical ocean salinity values (>24.70‰), liquid water does not have a temperature of maximum density This has large repercussions for freezing of ocean water versus fresh water (as seen in the related fresh water homework values changes with increasing salinity problem) OC3230-Paduan images Copyright © McGraw Hill Chap 5-6: Seawater At typical ocean salinity values (>24.70‰), liquid water does not have a temperature of maximum density Ocean ranges of temperature and salinity are relatively narrow Look ahead: this is an example of a T-S diagram OC3230-Paduan images Copyright © McGraw Hill Chap 5-6: Seawater OC3230-Paduan images Copyright © McGraw Hill Chap 5-6: Seawater Homework Problem related to water density, freezing points, and heat capacity Problem set and related MATLAB library of seawater density routines can be downloaded from the course web site OC3230-Paduan images Copyright © McGraw Hill Chap 5-6: Seawater How is salinity measured? 1) Absolute Salinity: Silver Nitrate Titration method to determine Chlorinity: SA (‰) = 1.80655 x Cl 2) Practical Salinity Method based on conductivity (CTD=cond.temperaturedepth) Must continuously check CTD calibration against conductivity of “Wormly Water” OC3230-Paduan Must measure T very accurately along with conductivity to get S images Copyright © McGraw Hill Chap 5-6: Seawater Dissolved Gases Mixing with atmos. + photosynthesis Animal respiration Sinking from above + decreased respiration OC3230-Paduan images Copyright © McGraw Hill Chap 5-6: Seawater Dissolved Gases Deep basins with little physical mixing, due to (or resulting in) strong vertical density stratification, can lead to anoxic conditions e.g., Black Sea, some fjords, some trenches OC3230-Paduan Mixing with atmos. + photosynthesis Animal respiration images Copyright © McGraw Hill Chap 5-6: Seawater The Equation of State The relationship between Temperature, Salinity, Pressure and the resulting density, i.e., = f(T,S,P) Recall in the atmosphere (in an ideal gas): P = RT P also, P T Related: Potential Temperature = that temperature a water parcel would have if it were raised from its actual depth to some reference depth adiabatically (without exchanging heat) Computation requires knowledge of “adiabatic lapse rate” Several online calculators are available to give = f(T,S,P,Pref); usually = f(T,S,P,0) OC3230-Paduan images Copyright © McGraw Hill Chap 5-6: Seawater Potential Temperature, , is less than or equal to the in situ (i.e., observed) temperature, T Difference is not significant in shallow waters (above ~500m) OC3230-Paduan images Copyright © McGraw Hill Chap 5-6: Seawater What about the equation of state for seawater? It is very complicated and cannot be expressed by a simple formula An empirical formula has been developed and agreed upon by the international community OC3230-Paduan images Copyright © McGraw Hill Chap 5-6: Seawater Empirical Equation of State for sea water (EOS90); Expressed in terms of the surface density and K OC3230-Paduan images Copyright © McGraw Hill Chap 5-6: Seawater Conclusion: Equation of State for sea water is complex but empirical formulae give give us consistent results given T,S,P Next: Sound in the Ocean T S P The secant bulk modulus, K, is related to the compressibility of sea water and, thereby, to the speed of sound OC3230-Paduan images Copyright © McGraw Hill Chap 5-6: Seawater Sound in the Ocean The speed of sound, c, is related to the density and the compressibility, , of the medium according to: where, K = 1/ is the bulk modulus c 1 OC3230-Paduan images Copyright © McGraw Hill Chap 5-6: Seawater Sound in the Ocean The speed of sound, c, is related to the density and the compressibility, , of the medium according to: where, K = 1/ is the bulk modulus c 1 Sound travels as a “Longitudinal” or compression wave: y x To be compared with “Transverse” waves (i.e., surface waves): z x Animations courtesy of Dr. Dan Russell, OC3230-Paduan Kettering University images Copyright © McGraw Hill Chap 5-6: Seawater Sound in the Ocean The speed of sound, c, is related to the density and the compressibility, , of the medium according to: where, K = 1/ is the bulk modulus c 1 Note that c is inversely proportional to compressibility; While water is much less compressible than air, sound travels through water much more quickly than it does through air water << air OC3230-Paduan cwater >> cair cwater ~ 1500 m/sec cair ~ 330 m/sec images Copyright © McGraw Hill Chap 5-6: Seawater Sound in the Ocean The speed of sound, c, is related to the density and the compressibility, , of the medium according to: where, K = 1/ is the bulk modulus c 1 Note that c is inversely proportional to compressibility; While water is much less compressible than air, sound travels through water much more quickly than it does through air water << air cwater >> cair cwater ~ 1500 m/sec cair ~ 330 m/sec Given c, the wavelength, , and frequency, f, of sound waves (seawater) f are related by the wave equation: Low Frequency 30Hz 50m High Frequency 1.5MHZ 1mm c = f Instruments: OC3230-Paduan 10-100kHz 14-1.4cm images Copyright © McGraw Hill Chap 5-6: Seawater Sound in the Ocean Sound speeds in the ocean are ~1500 m/sec but they vary by about 5% as a function of T,S, and P: T c S c P c Variations in c lead to bending (turning) of sound waves according to Snell’s Law sin 1 c1 sin 2 c 2 Snell’s Law applies equally to surface waves and light waves; Animation courtesy of Dr. Dan Russell, OC3230-Paduan Kettering University If c changes in the medium, wave crests will bend toward the region of slower speed images Copyright © McGraw Hill Chap 5-6: Seawater Sound in the Ocean Sound waves spreading out uniformly for c = constant Bending upward toward lower c Bending downward toward lower c Animations courtesy of Dr. Dan Russell, OC3230-Paduan Kettering University images Copyright © McGraw Hill Chap 5-6: Seawater Sound in the Ocean The distribution of c can lead to shadow zones where sound energy does not penetrate OC3230-Paduan images Copyright © McGraw Hill Chap 5-6: Seawater Sound in the Ocean A more complicated distribution of c(z) and resulting shadow zones The process of bending or turning of waves due to variations in their speed is called “refraction” SOFAR: Sound Fixing and Ranging channel; energy is trapped by the effect of minimum c around 1000m OC3230-Paduan images Copyright © McGraw Hill Chap 5-6: Seawater Sound in the Ocean SOFAR: Sound Fixing and Ranging channel; energy is trapped by the effect of minimum c around 1000m OC3230-Paduan images Copyright © McGraw Hill Chap 5-6: Seawater Sound in the Ocean There is no middepth minimum or sound channel at high latitudes Overview: Temperature and Pressure are most critical to c Salinity is important at high latitudes Look Ahead: Global distributions of T(z), S(z) OC3230-Paduan images Copyright © McGraw Hill Chap 5-6: Seawater Sound in the Ocean Match the sound speed profiles on the left with the refraction and shadow zone results on the right OC3230-Paduan images Copyright © McGraw Hill Chap 5-6: Seawater Sound in the Ocean Match the sound speed profiles on the left with the refraction and shadow zone results on the right OC3230-Paduan images Copyright © McGraw Hill Chap 5-6: Seawater Sound in the Ocean Absorption Coefficient versus frequency Attenuation: 1) Spreading (Spherical vs Cylindrical) “Relaxation” 2) Absorption, I = Ioe-ax (energy transfer to medium; a=absorption coefficient) 3) Scattering (redirection by particles) OC3230-Paduan f a I Lower frequencies travel further images Copyright © McGraw Hill Chap 5-6: Seawater Sound in the Ocean Target Strength (active acoustic applications) Related to effectiveness of the reflection of sound Measured by the Acoustic Impedance, Z = c The Reflectivity, R, determines how much acoustic energy is reflected from a discontinuity and how much is transmitted through it; R = (Z1 - Z2)/(Z1 + Z2) x 100% closely matched impedance values OC3230-Paduan images Copyright © McGraw Hill Chap 5-6: Seawater Sound in the Ocean Ambient Noise (passive acoustic applications) Related to background sound Environmental processes make distinctive noises Can cause a detection problem for some applications Can also be inverted to remotely monitor the environment (e.g., rain rate, wind speed) OC3230-Paduan images Copyright © McGraw Hill Chap 5-6: Seawater Sound in the Ocean Acoustic Thermometry of Ocean Climate (ATOC) Exploits SOFAR channel to send sound great distances Travel time is tied to the average T along the path OC3230-Paduan images Copyright © McGraw Hill