Virus Genome Polarity Segments Morphology Enveloped Diseases
advertisement
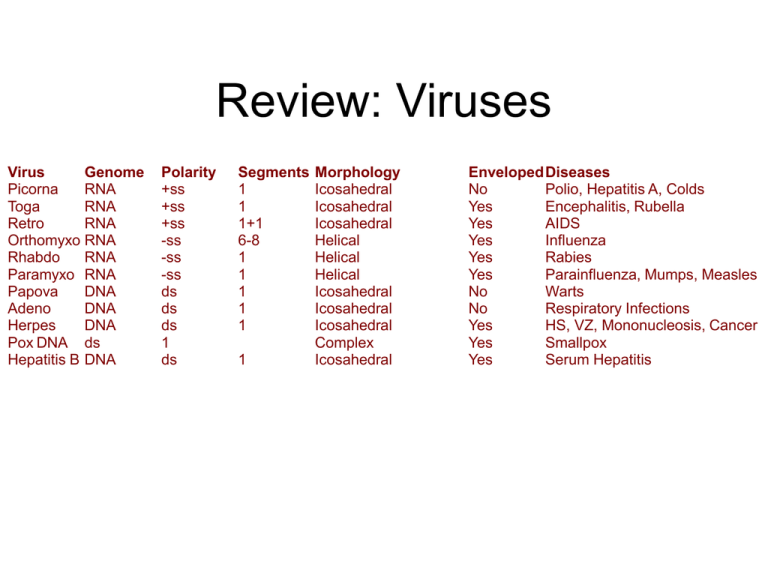
Review: Viruses Virus Genome Picorna RNA Toga RNA Retro RNA Orthomyxo RNA Rhabdo RNA Paramyxo RNA Papova DNA Adeno DNA Herpes DNA Pox DNA ds Hepatitis B DNA Polarity +ss +ss +ss -ss -ss -ss ds ds ds 1 ds Segments 1 1 1+1 6-8 1 1 1 1 1 1 Morphology Icosahedral Icosahedral Icosahedral Helical Helical Helical Icosahedral Icosahedral Icosahedral Complex Icosahedral EnvelopedDiseases No Polio, Hepatitis A, Colds Yes Encephalitis, Rubella Yes AIDS Yes Influenza Yes Rabies Yes Parainfluenza, Mumps, Measles No Warts No Respiratory Infections Yes HS, VZ, Mononucleosis, Cancer Yes Smallpox Yes Serum Hepatitis Encephalitis and Meningitis • Encephalitis is a brain inflammation that causes sudden fever, vomiting, headache, light sensitivity, stiff neck and back, drowsiness, and irritability. Meningitis is an infection that causes inflammation of the meninges that surround the brain and spinal cord. Symptoms of meningitis include high fever, headache, nausea, vomiting, and stiff neck. Encephalitis and Meningitis • Encephalitis is an inflammation of the brain. There are many types of encephalitis, most of which are caused by infections. Most often these infections are caused by viruses. Encephalitis can also be caused by diseases that cause inflammation of the brain. Encephalitis and Meningitis • Meningitis is an inflammation of the membranes (called meninges) that surround the brain and spinal cord. Meningitis may be caused by many different viruses and bacteria. It can also be caused by diseases that can trigger inflammation of tissues of the body without infection (such as systemic lupus erythematosus and Behcet's disease). Antiviral Agents: Influenza http://microbiology.mtsinai.on.ca/presentations/mazzulli/ mazzulli1.html Review: Chemotherapeutic Agents to Treat Viral Infections • Control of Viruses Since viruses lack the structures and metabolic processes that are altered by common antibiotics, antibiotics are virtually useless in treating viral infections.To date, only a few chemotherapeutic agents have been found to be somewhat effective against just a few limited viruses. Antiviral Drugs (general) • 1. amantadine (Symmetrel): used prophylactically against influenza A in high-risk individuals. • 2. rimantidine (Flumadine): used for treatment and prophylaxis of influenza A. Mechanism of Action of Amantadine and Rimantadine • These agents were discovered by random screening and are now known to interfere with a viral ion channed called matriz (M2) protein. • This causes an inhibition of uncoating of the virus. • At high concentrations, they also buffer the pH of the endosomes and prohibit the acidic environment needed for Hemaglutanin (HA) to fuse the viral membrane with that of the endosome. • • • • • • Structure and mechanism of the M2 proton channel of influenza A virus Jason R. Schnell1 & James J. Chou1 Department of Biological Chemistry and Molecular Pharmacology, Harvard Medical School, Boston, Massachusetts 02115, USA Correspondence to: James J. Chou1 Correspondence and requests for materials should be addressed to J.J.C. (Email: james_chou@hms.harvard.edu). Top of page Abstract The integral membrane protein M2 of influenza virus forms pH-gated proton channels in the viral lipid envelope1. The low pH of an endosome activates the M2 channel before haemagglutinin-mediated fusion. Conductance of protons acidifies the viral interior and thereby facilitates dissociation of the matrix protein from the viral nucleoproteins—a required process for unpacking of the viral genome2. In addition to its role in release of viral nucleoproteins, M2 in the trans-Golgi network (TGN) membrane prevents premature conformational rearrangement of newly synthesized haemagglutinin during transport to the cell surface by equilibrating the pH of the TGN with that of the host cell cytoplasm3. Inhibiting the proton conductance of M2 using the anti-viral drug amantadine or rimantadine inhibits viral replication4, 5, 6, 7. Here we present the structure of the tetrameric M2 channel in complex with rimantadine, determined by NMR. In the closed state, four tightly packed transmembrane helices define a narrow channel, in which a 'tryptophan gate' is locked by intermolecular interactions with aspartic acid. A carboxyterminal, amphipathic helix oriented nearly perpendicular to the transmembrane helix forms an inward-facing base. Lowering the pH destabilizes the transmembrane helical packing and unlocks the gate, admitting water to conduct protons, whereas the Cterminal base remains intact, preventing dissociation of the tetramer. Rimantadine binds at four equivalent sites near the gate on the lipid-facing side of the channel and stabilizes the closed conformation of the pore. Drug-resistance mutations are predicted to counter the effect of drug binding by either increasing the hydrophilicity of the pore or weakening helix–helix packing, thus facilitating channel opening. Nature 451, 591-595 (31 January 2008) | doi:10.1038/nature06531; Received 15 July 2007; Accepted 3 December 2007 Antiviral Drugs (general) • 3. zanamivir (Relenza): used to limit the duration of influenza A and B infections. • 4. oseltamivir (Tamiflu): used limit the duration of influenza infections. OH OH OH O COOH O O COOEt O N H HN NH NH2 Zanamivir (Relenza) NH NH2 Oseltamivir (Tamiflu) Two different therapeutic approaches for treating: A. A DNA virus (e.g. Herpes) B. An RNA virus (e.g. Influenza) Neuramindase is an enzyme which is capable of cleaving the sialic acid sugar moiety from selected glycoproteins and glycolipids on the surface of infected cells. This cleavage promotes the release of progeny virus from infected cells. The neuraminidase enzyme • • • • • • Neuraminidase is attached to the viral surface by a single hydrophobic sequence of 29 amino acids The enzyme can be easily mutated. There are two main types corresponding to influenza A and B. However, the active site is located in a deep pocket and the 18 amino acids making up the active site itself are constant. The enzyme is critical to the infective process, particularly including preventing viral aggregation or binding to hemaglutinin or inactivation by respiratory mucous. It is essential for proper liberation (shedding) of the new virus. Both neuraminidase (NA) and hemaglutanin (HA) act as antigens for flu vaccines. However, due to the frequency with which influenza A mutates these proteins, new flu vaccines are required each year. Transition state analogs • An early search for inhibitors of neuraminidase was unsuccessful. • Once the crystal structure was available, it was decided to search for transition state analogs. • Recall that the active site of the enzyme will bind and stabilize the transition state more effectively than it will stabilize the substrate itself, thus resulting in an overall decrease in activation energy for the chemical transformation.






