lides for Lecture 16: October 26th and 29th, 2007
advertisement

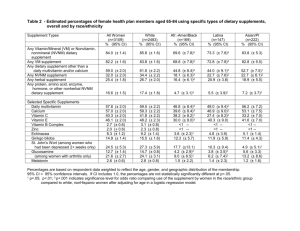
Supplement Industry and Regulations What are dietary supplements? Powdered Protein? Testosterone? Nutra-sweet? EPO? Androstenedione? Human Growth Hormone? Calcium? Vitamin C? Jellybeans? Supplements are NOT… Foods Food Additives Drugs "Food" means a raw, cooked, or processed edible substance, ice, beverage, or ingredient used or intended for use or for sale in whole or in part for human consumption, or A food additive is any substance added to food. Legally, they are "any substance of which the intended use results or may reasonably be expected to resultdirectly or indirectly-in its becoming a component or otherwise affecting the characteristics of any food." This includes any substance used in production, processing, treatment, packaging, transportation or storage of food. Food additives must be approved by the FDA. Drugs alter physiological and biological processes. According to the 1985 Federal Food, Drug, and Cosmetic Act, a drug is “intended for diagnosis, cure, mitigation, treatment or prevention of disease” and “articles (other than food) intended to affect the structure or function of the body”. Before a drug can be sold in the US it must undergo clinical studies to determine its efficacy, safety, possible interactions with other drugs, and appropriate dosage. Drugs must be approved by the FDA. chewing gum. Foods must be handled and labeled in accordance with laws of the FDA. How are Supplements defined? Supplement = product that supplies a component missing in diet. Since 1994 (DSHEA), definition is: 1. A product (not tobacco) intended to supplement the diet that contains: a vitamin, a mineral, a dietary substance to increase total daily intake, a concentrate, a metabolite, a constituent, an extract, a combination of the above. 2. Intended for ingestion in pill, capsule, tablet or liquid form. 3. Not for use as a conventional food or as the sole item of a meal or diet. 4. Labeled as a dietary supplement. 5. Includes products such as approved drugs or antibiotics that were a dietary supplement prior to approval. DSHEA: Dietary Supplement Health and Education Act Love it or hate it… KNOW IT! • Passed originally in Congress in 1994 • Revised in 1999 • Places the “burden of proof” on the FDA to ensure product safety. • Created limits on what claims could be made about dietary supplements. WHY DSHEA EXISTS • If you were Congress, would you regulate dietary supplements like: – Food? – Drugs? – Some sort of in between “natural” consumable substance? • How much money are we talking about? In 2004, 18.9 percent of Americans reported that they had taken one or more dietary supplements in the past year. Marketing a new drug requires a complex series of testing in both animals and humans to determine: 1. safety 2. effectiveness 3. possible interactions with other substance 4. appropriate dosages Takes millions of dollars and years of research to bring a new drug (or food additive) to market. Prozac, Viagra, Olestra, NutraSweet Who is responsible for supplement safety and efficacy? The FDA (Food and Drug Administration) oversees safety, manufacturing and product information (such as claims) in package inserts, on the label, or in accompanying literature. FTC (Federal Trade Commission) oversees advertising (TV, newspaper, magazine, internet?) The Dietary Supplement Health and Education Act (DSHEA) of 1994 specifies powers that the FDA has to regulate the sale of dietary supplements. The consumer??? Recently revised (1999), DSHEA now requires that supplement labels must prominently display the following information: 1. That they are a dietary supplement 2. Statement of identity (e.g. ginseng) 3. Quantity (e.g. 60 capsules) 4. Serving size, amount of active ingredients, list of ingredients in descending order of quantity. Cannot make any claim to prevent, treat, cure or mitigate a disease OR have treatment implied in the name (e.g. “cardiocure”) OPTIONAL: structure/function claims CAN make general claims about a nutrient or other compound in terms of a non-specific effect on health and well-being: “maintains bone health”; “supports the immune system” Requires label to say: “This statement has not been evaluated by the FDA. This product is not intended to prevent, treat, cure or mitigate disease” Manufacturers (CRN) have lobbied hard to soften the FDA position on health claims with some success. Manufacturers can make 3 “claims” about their products: Nutrient content claims Describes the level of a nutrient in a food or dietary supplement. For example, a supplement containing at least 200 milligrams of calcium per serving could carry the claim "high in calcium." Show a link between a food or substance and a Disease claims disease or health-related condition. FDA authorizes (needs FDA these claims based on a review of the evidence. approval) Example, “calcium lowers the risk of osteoporosis”. Structure – function claims Can make general claims about a nutrient or other compound in terms of a non-specific effect on health and well-being. Example, “calcium builds strong bones”. Manufacturers may base claims on their review of the literature. These claims do not need FDA authorization but must include “This statement has not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease”. Section 6 of DSHEA NO DISEASE CLAIMS but statements of nutritional support and “Structure/Function” Claims are allowed 4 general categories of S-F claims: 1. Nutrient deficiency disease - but must also disclose its prevalence 2. Role of a nutrient / ingredient in affecting a structure or function 3. Describes the documented mechanism by which a nutrient or dietary ingredient acts to maintain a bodily structure/function 4. General well-being UNACCEPTABLE: DISEASE (DRUG) ACCEPTABLE: STRUCTURE FUNCTION Protects against heart disease Helps maintain cardiovascular function Lowers cholesterol level Promotes healthy cholesterol level Reduces pain of arthritis Promotes healthy joints Prevents urinary tract infections Promotes urinary tract health Summary Difference between a supplement and a drug... - Why do we care? Testing and marketing by the FDA is different for supplements compared to drugs (and food& food additives). DSHEA (act signed by Clinton in 1994): basically allows supplement manufacturers to market more products as supplements and to provide more information to the consumer about the supplement’s benefits. - In the eye’s of the consumer, it weakened the enforcement ability of the FDA. Because of the DSHEA... - supplement labels are more “consumer friendly” - But are supplements still “safe”? Supplements used to regulated as food additives but getting FDA approval for new food additives takes years of research...$$$ “Food additive” does not apply to supplements... They are not subject to the same pre-market safety assessment. With the DSHEA, FDA regulation went from evaluating pre-market safety to policing the industry This means that the FDA evaluates adverse event reports and then makes decisions about whether or not to categorize supplements as adulterated (impure or questionable). Supplement Regulation: Legal in the USA... - Each state reserves the right to ban the sale of a substance (ex: ephedra) - Other countries have different supplement regulations. Legal for sale doesn’t mean legal in competition... - Athletic governing bodies vary in their list of banned substances. - Many banned substances are legal over-thecounter drugs and dietary supplements. “Consumer Savvy” • Because the DSHEA requires less premarket review for dietary supplements making an informed decision as a consumer is important. – Look for unbiased information and evaluate studies as we have practiced in class. – Also look to see if the findings of a study have been re-produced... Tips for developing sport “supplement savvy” – Be suspicious of a single substance that claims to do multiple things “build muscle, increase strength, burn fat and increase stamina...etc” – Be wary if companies tell you that you don’t have to eat properly when taking the supplement. – Natural doesn’t equal safe – Be critical of the studies that the manufacturer cites as proof of safety/efficacy (mistrust the information if you can’t find the reference!) – Use common sense, if the claim appears too good to be true, it probably is. The R&D Conundrum The R&D Conundrum Fast Cheap Good Good + Cheap = Not fast Good + Fast = Not cheap Fast + Cheap = Not good Is it better to be FIRST? Is it better to be BEST? In the supplement industry, enough research is... Enough to convince enough consumers to buy the product that $$ from sales greatly exceeds the costs of manufacture, distribution and advertising. $$$$ in >>> $ out To do more violates the interests of employees, shareholders and, in terms of price, consumers. Doing more than the minimum research needed to maximize sales is not only unnecessary but even incompatible with the interests of the company. For academic scientists, enough research is ….. First: efficacy (does it work?) safety (does it harm?) but also: We are charged with understanding context mechanism of action, impact on endogenous production,effects on other metabolic pathways etc. Doing less than the minimum research required to understand the physiological context is incompatible with our responsibilities as scientists Can’t we all just get along? YES, in the sense that we share the same basic goals of optimizing safety, health, and performance NO, in the sense that we have fundamental disagreements about who (target population), what (top priorities), why (knowledge/sales), when (how soon), and how (single study vs. line of research ) Consumer bottom line = DO YOUR HOMEWORK BEFORE BUYING A SUPPLEMENT!