Rosa alba
advertisement

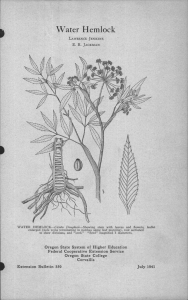
Medicinal Chemistry But First – Some Flowers 15th Century Turkish Tulip Semper Augustus Tulip Triumph Tulips Queen of the Night Tulip Rosa alba – White Rose of York – England pre-16th Century Madame Hardy Rose – bred 1832 Modern Hybrid Tea Rose Medicinal Chemistry • Medicinal chemistry is the chemical study of chemical substances useful in medical treatments Diospyros lycioides – source of chewing sticks in Namibia Ceanothus americanus – Native American chewing stick Modern Chewing Sticks • Most chewing stick plants have a wide range of antibacterial activity against a number of odontopathic bacterial species, and many also contained healing and/or analgesic compounds Bloodroot – Sanguinaria canadensis Rhizome of Bloodroot Bloodroot extracts to treat dental plaque • Bloodroot extracts have been identified as potentially valuable in controlling plaque • Blood root has many alkaloids, known as sanguinaria alkaloids, and sanguinarine in particular, is thought to be a potential problem limiting the usefulness of blood root as a dental medicine • There is an indication that sanguinarine may provoke glaucoma in predisposed humans and cats. Double Blind Testing • A key component of western testing is to do double-blind testing, so neither healer nor patient knows what the patient is receiving. • Such tests often involve the use of a real substance and a placebo. • The test for the placebo effect assumes that patients are not expecting the substance to have certain effects. • If patients do expect certain effects, it renders placebo testing difficult or impossible. Dwarf ginseng – Panax trifolium Medicinal properties of Ginseng • There are many claims that ginseng (Panax sp. – F. Araliaceae – the arums) increases sexual functioning, has anti-cancer properties, boosts the immune system, and even increases ability to perform in stressful situations. • Difficult to test because ginseng preparations differ in their composition. • Many of the chemicals produced by ginseng have counteractive effects. Isolated compounds work well in vitro, but simple ginseng preparations in vivo do not seem to have the benefits originally claimed. How ginsenosides are absorped, transported, degraded, and excreted is poorly known in humans. Opium poppy – Papaver somniferum Plant chemical composition changes with development • The opium poppy Papaver somniferum is a good example of changes in metabolites during development. The seeds are free of alkaloids. After germination, the plant produces narcotine within 3 days. When the seedling is about 7 cm tall, the plant begins to produce codeine, morphine, and papaverine. Total alkaloid content slowly increases until flowering and then there is a sharp increase in alkaloids until the floral leaves fall off the plant. Poison hemlock – Conium maculatum Poison hemlock in the wild Water hemlock – Cicuta virosa Poison Hemlocks • Poison hemlock and the water hemlocks are the most poisonous plants in North America. The active ingredient in poison hemlock is the alkaloid coniine. It is a central nervous system stimulant that affects the body like a nicotine overdose; paralysis creeps from the lower limbs upward. Death is due to the paralysis of the diaphragm and subsequent respiratory failure. Water hemlocks are toxic due to an alcohol, cicutoxin. It produces violent convulsions and death. Alkaloid content varies during the day • In poison hemlock, the amount of coniine varies during the day. The fruits are very high in coniine at 4 a.m. (226 micrograms) and low in coniine by 4 p.m. (8 micrograms). The amount of coniine varies with amount of sun as well. Another relative, wild parsnip (Pastinaca sativa) is toxic to the skin and is more toxic during bright sun than in cloudy weather or at night. Wild parsnip – Pastinaca sativa Wild parsnip in the field Teonanacatl – The Flesh of the Gods Psilocybe mushrooms Dr. Albert Hoffman – Swiss Chemist Conocybe – also produces psilocin Panaeolus – also produces psilocin, may be toxic Stropharia – also produces psilocin, may be toxic Chemical structures of several hallucinogenic substances Drug Similarities • Interestingly, the drug Visken, used to treat hypertension was developed as an analog to 4hydroxyindole, the phenolic nucleus of psilocybin and psilocin, from chemicals in Albert Hoffman’s lab.