Unit 11 Solid Liquid Heat
advertisement

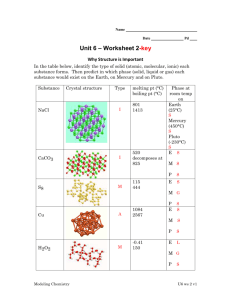
UNIT IX SOLIDS, LIQUIDS HEAT PROBLEMS CHAPTER 16 PART1 AND CHAPTER 14 INTERMOLECULAR FORCES Forces between molecules Not as strong as within molecules (covalent and ionic) van der Waals Forces (Intramolecular Force) Dispersion Forces (London Forces) Exists between non-polar molecules weakest I.M.F. Due to temporary shifts in electron cloud density Examples CH4 O2 Dipole-Dipole Forces • slightly polar • Example: CHCl3 HYDROGEN BONDING • VERY polar • Strongest • Examples NH3 (N -- H) H2O (O -- H) HF (F-- H) HCl (Cl -- H) SOLIDS AND LIQUIDS SOLIDS Orderly rigid and cohesive Particles that vibrate around fixed points SOLIDS CRYSTAL • true solids • particles are arranged in an orderly repeating 3-D pattern SOLIDS CRYSTALS (cont) – consists of a MEMBER o one particle (ion, atom, molecule SOLIDS several members together make up UNIT CELL simplest repeating unit retains its shape SOLIDS several unit cells together make up a CRYSTAL LATTICE 3-D arrangement of unit cells repeated over and over SOLIDS VocabANHYDROUS (without water) - compound containing no water of hydration HYDRATE-compound with water molecules attached (CuSO4 * 6H2O) SOLIDS AMORPHOUS – solid – no definite repeating pattern – no true melting point – no plateau EXAMPLES: glass, butter, tar, plastic LIQUIDS DEFINITION – particles vibrate around a moving point – non-orderly, non-rigid, cohesive – more space between particles than a solid – exert a vapor pressure – Fluid – ability to flow LIQUIDS UNITS Temperature average kinetic energy (KE) °C °F K (Kelvin) LIQUIDS VAPOR PRESSURE Definition pressure exerted by vapor molecules above a liquid when dynamic equilibrium is reached LIQUIDS Pressure measure of force with which gas molecules hit the side of container normal atmospheric pressure at sea level Standard Pressure Units =760 torrs = 760 mmHg = 101.3 kilopascals (kPa) LIQUIDS VAPOR PRESSURE Dynamic equilibrium - 2 opposite processes occurring at same time and same rate VAPOR LIQUID LIQUIDS VAPOR PRESSURE Dynamic Equilibrium depends upon: Temperature increase temperature, increase vapor pressure VAPOR T VP LIQUID LIQUIDS • • Strength of inter-molecular forces; hydrogen bonding(such as water) is strongest. increase forces; decrease vapor pressure VAPOR VAPOR LIQUID LIQUID WATER ALCOHOL IMF VP LIQUIDS Viscosity measure of resistance to flow (how thick) Example – Molasses (syrup) has a high viscosity Volatility - how easily a liquid evaporates LIQUIDS Very volatile: high vapor pressure low IMF low boiling point EXAMPLES: alcohol, perfume VAPORVAPOR LIQUID ALCOHOL LIQUIDS Not volatile low vapor pressure high IMF high boiling point Examples: molasses, water VAPOR VAPOR LIQUID WATER CHANGES IN STATE OR PHASES Sublimation– solid changes directly into gas without going through the liquid state Examples: solid iodine, solid air fresheners, "dry" ice CHANGES IN STATE OR PHASES Melting / Freezing – goes from solid to liquid or liquid to solid CHANGES IN STATE OR PHASES Vaporization • evaporation occurs only on the surface at room temperature cooling process Sweat • boiling occurs throughout the liquid requires energy CHANGES IN STATE OR PHASES Boiling Point: • vapor pressure = atmospheric (outside) pressure (for any boiling point) • normal boiling point vapor pressure = standard pressure standard pressure = 1 atm, 760 torrs, 760 mm Hg,101.3 kPa Boiling Point: • different altitudes higher altitudes have lower air pressures Denver has a lower boiling point 95 °C than Houston has (100 °C) Foods take longer to cook in Denver than Houston. VAPOR PRESSURE DIAGRAMS 1000 900 800 760 700 600 500 400 300 200 100 20 40 60 Temperature ( °C) 80 100 PHASE DIAGRAMS Graphs that show conditions (temperature and pressure) under which a substance will exist as a solid, liquid, or gas. PHASE DIAGRAMS Z 800 760 700 600 500 400 X - Triple point X-Y line - These Z Critical temp. and All three states are in are sublimation pressure. A gas can't equilibrium at this points. be liquified above this temperature and pressure. point. X 300 200 100 40 60 100 120 80 Temperature (°C) 140 160 180 PHASE DIAGRAMS Z 800 760 700 600 500 Lines represent 2 phases in equilibrium. LIQUID X 400 300 200 GAS 100 40 60 100 120 80 Temperature (°C) 140 160 180 PHASE DIAGRAMS Z 800 760 700 600 500 400 300 Normal boiling point (condensation) occurs here. when standard pressure crosses liquid / gas line X 200 100 40 60 100 120 80 Temperature (°C) 140 160 180 PHASE DIAGRAMS Z 800 760 700 600 500 400 300 Normal melting point (freezing) occurs where standard pressure crosses Normal melting liquid / solid line.point (freezing) occurs here X 200 100 40 60 100 120 80 Temperature (°C) 140 160 180 PHASE DIAGRAMS Z 800 760 700 600 500 400 X 300 200 100 40 60 100 120 80 Temperature ( °C) 140 160 180 UNIQUE PROPERTIES OF WATER STRONG HYDROGEN BONDING CAUSES: – high boiling point and melting point – high specific heat capacity – high surface tension needle floats – Water droplets are spherical HEAT VS. TEMPERATURE Energy transferred Average Kinetic from one body to Energy another because of a Written as KE difference in temperature HEAT VS. UNITS – calories (c) – kCal - C • (1000 calories) – Joules - J • energy for one heartbeat – 1 cal = 4.18 J – 1 kCal = 4180 J TEMPERATURE UNITS – °C - celsius – °F -Fahrenheit – K - kelvin (no degree sign!) HEAT VS. Measured by: – indirectly by a calorimeter TEMPERATURE Measured by: – thermometer HEAT VS. DEPENDS UPON – mass more mass means more heat – Cp (S) - specific heat type of matter some hold heat better than others – DT - change in temperature TEMPERATURE DEPENDS UPON – amount of movement of the particles in the substance HEAT VS. FORMULA q=energy (J) m=mass (g) q = (m) (DT) (Cp) q = (m) (T2-T1) (Cp) TEMPERATURE Specific Heat or Heat Capacity Amount of heat needed to raise 1 gram of a substance 1 degree Celsius Units – – (J/goC) (cal/goC) Examples – – – water --- 4.18 J/goC or 1 cal/goC Au --- 0.129 cal/goC alcohol --- 2.45 J/goC Calorie Amount of heat needed to raise one gram of water one degree of celsius It takes one calorie to raise one gram of water one degree of Celsius Heat of Fusion - Hf Amount of heat needed to melt one gram of a substance at its melting point Units (cal/g) Examples – – water (Hf) = 334 J/g or 76.4 cal/g Ag = 88 J/g HEAT OF VAPORIZATION - Hv Amount of heat needed to vaporize one gram of a substance at its boiling point Examples – water (Hv) = 2260 J/g or 539 cal/g – Pb = 858 J/g PHASE CHANGE DIAGRAMS TEMPERATURE ( C) WATER Heat of vaporization Boiling point- substance is becoming a liquid 100 Heat of fusion Melting point substance is becoming a liquid 0 GAS LIQUID SOLID HEAT (cal/g) OR TIME Heat Calculations - Formulas The state remains the same and there is no change in temperature. q= joules m=grams Cp=J/g or J/c q= (m) (Cp) q = Heat Example of Non-Changing State Melting/freezing at melting point Vaporizing/condensing at boiling point How much energy does it take to melt 55g of gold at its melting point? Cp = 64.5 J/g q= (m) (Cp) = (55g)(64.5 J/g) = 3547.5 J HEAT EQUATION One substance with a temperature change q = (m) (Cp) (T2-T1) q=joules (J) m= mass (g) Cp = specific heat capacity (J/g °C) (J/c °C) T2 = final temperature T1 = initial temperature HEAT EQUATION EXAMPLE ***Heating or cooling with no change in state*** How much energy is released as 33 g of solid silver cools from 95 °C to 60°C? Cp of silver = 0.236 J/g °C HEAT TRANSFER EQUATION How a substance changes the temperature of another substance used in calorimeter calculations Energy LOST = Energy GAINED (m1) (Cp1) (T2-T1) = (m2) (Cp2) (T2-T1) Warm substance losing energy Cool substance gaining energy HEAT TRANSFER EQUATION EXAMPLE A piece of metal is dropped into a beaker of o boiling water whose temperature is 95 C. The 5g piece of metal is put into 100g of cold water at 20 oC. The temperature of the water rises to 30 oC. What is the specific heat of the metal? o Cp(water) = 4.18 J/g C EQUATION FOR CHANGING TEMPERATURE AND STATES Draw the phase change diagram TEMPERATURE ( C) CHANGING STATES AND TEMPERATURE 100 0 Use the following equations: q = (m) (Cp) q = (m) (Cp) (T2-T1) HEAT (cal/g) OR TIME TEMPERATURE ( C) CHANGING STATES AND TEMPERATURE 100 1. Heat solid to melting point 0 q = (m) (Cp) (T2-T1) HEAT (cal/g) OR TIME TEMPERATURE ( C) CHANGING STATES AND TEMPERATURE 100 2. Melting solid to liquid 0 q = (m) (Cp) HEAT (cal/g) OR TIME TEMPERATURE ( C) CHANGING STATES AND TEMPERATURE 100 3. Heat liquid to boiling point q = (m) (Cp) (T2-T1) 0 HEAT (cal/g) OR TIME TEMPERATURE ( C) CHANGING STATES AND TEMPERATURE 4. Change liquid to gas 100 q = (m) (Cp) 0 HEAT (cal/g) OR TIME TEMPERATURE ( C) CHANGING STATES AND TEMPERATURE 5. Heating gas 100 q = (m) (Cp) (T2-T1) 0 HEAT (cal/g) OR TIME CHANGING STATES AND TEMPERATURES When to use which equations: 1. Heat solid to melting point : q = (m) (Cp) (T2-T1) KE 2. Melt solid to liquid: q = (m) (Cp) PE 3. Heat liquid to boiling point: q = (m) (Cp) (T2-T1) KE 4. Change liquid to gas: q = (m) (Cp) PE 5. Heat gas: KE q = (m) (Cp) (T2-T1) CHANGING TEMPERATURE AND CHANGING STATES EXAMPLE How much energy is needed to change 30g of ice at -5 °C to steam at 120 °C?