Separation Science
advertisement

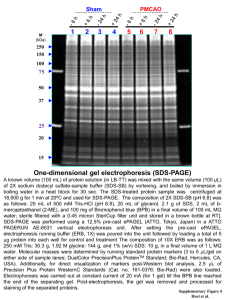
Biophysical Chemistry Gel Electrophoresis Definition • Electro = Charge + Phorsesis= Carry • Electrophoresis = Separation of charged molecules by differences in their rate of migration in an electric field. The Components of The System • Molecules to be separated – Proteins – Nucleic Acids • Support medium – Gel (Starch, Polyacrylamide or Agarose) • Buffer System – High Buffer Capacity • DC Power Source – 50 – 1000 V Common Support Media for Biological Molecules • Proteins – Native Gel (Acrylamide or Starch) – Denaturing (SDS) Gel (Acrylamide) • Nucleic Acids – DNA & RNA – Agarose Gel – Acrylamide Gel Factors that Influence Mobility • Properties of the Molecules to be separated – Molecular size (MW) – Molecular shape – Molecular charge • Properties of the System – – – – Electric field strength (V/cm) Porosity of the support medium (% S) Conductivity of the buffer (R) pH of the buffer These Factors Interact • Mobility is proportional to charge/MW. – Charge is affected by buffer Ph • Mobility is proportional to field strength. – Allowable field strength is affected by buffer conductivity (high conductivity high current heat) • Mobility is inversely proportional to buffer conductivity. Making a Separation • Electrophoresis systems are designed to optimize the separation of specific molecule types based on specfic molecular parameters: – Nucleic acids: Charge/BP is a constant. Separation can be based on number of base pairs (given all molecules have same shape). Larger molecules move slower due to friction with gel – Proteins: Charge varies as a function of amino acid composition and buffer pH. Separation is based on Charge/MW (shape may also vary). The exact combination of factors varies for each molecule Separating Proteins Based on MW • The problem associated with protein separation (too many separation parameters) has been solved by using denaturing SDS Gels – The proteins are heat denatured which makes them all the same shape (linear) – The proteins are coated with an ionic detergent (SDS) which gives all molecules approximately the same overall negative charge – Separation is based on MW alone SDS Polyacrylamide Gel Electrophoresis (SDS PAGE) Separation of DNA Molecules in Agarose Gels • In most cases the molecules are linear • The phosphate groups bear negative charge at neutral pH (2 phosphates/BP) • Therefore mobility will be based on number of base pairs/molecule • The Procedure for making and running agarose gels is shown in the following video Physics Concepts Addressed • Basic DC circuits and Ohm’s law. – Ionic strength and conductivity – Ohmic heating • The concept of an electrical field. – Force acting on a charged molecule as a result of an applied voltage • Frictional resistance to mobility – Friction as a function of molecular surface area • The use of logarithmic paper (or spread sheets). – Determination of molecular size Practical Considerations for Teaching Gel Electrophoresis in the High School • Availability of materials and equipment – Science Kit & Carolina both have kits for doing DNA electrophoresis. • Cost (Demonstration vs. Hands on) • Safety issues – Toxic chemicals – minimal toxicity except for ethidium bromide stain. Can not be used!!! – Electrical hazards- 80 V DC is the standard voltage used in most setups. The power supplies in the kits are often limited to 100 V. GFI outlets are manditory. References and Web Sites • • • • • • • • • Polyacrylamide Gel Electrophoresis of Proteins and DNA-Modern Bio. Inc http://www.modernbio.com/polyacrimide.htm Simple Electrophoresis of dyes http://gslc.genetics.utah.edu/units/activities/electrophoresis/ Vivoy et al. The physics of DNA Electrophoresis. Contemp Phys. 33 1-40 (1992) Physics and gel electrophoresis: using terminal velocity to characterize molecular weight http://www.iop.org/EJ/abstract/0143-0807/19/6/011 Edvoteck http://www.southernscientific.com/apbiology_electrophoresis.asp Wards http://wardsci.com/search.asp?t=ss&ss=Electrophoresis&x=14&y=8 Nat Cent Biotech Edu. http://www.ncbe.reading.ac.uk/NCBE/MATERIALS/DNA/baseunit.html Physics at Work (Biophysics) http://www.phy.cam.ac.uk/camphy/outreach/physics_at_work_2003/exhibito r/biophysics.htm Low toxicity stains http://www.bioscience-explained.org/EN1.2/schollar.html