File - Dr. Luna -
advertisement

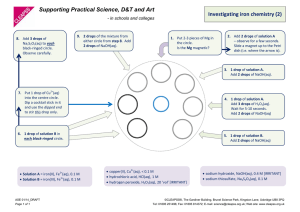
Physical and Chemical Changes Lab Part 1 – Food Coloring 1. Add 100ml of water to a clear cup 2. Add 2 drops of food coloring to the water in the cup. DO NOT STIR! 3. Fill in your data table with your observations. 4. Determine if a chemical or physical change occurred and explain why. Part 2 – Silver nitrate (AgNO3) with sodium hydroxide (NaOH)? 1. Add 2 drops of AgNO3 to a well. 2. Add 2 drops of NaOH to the same well. 3. Fill in your data table with your observations. 4. Determine if a chemical or physical change occurred and explain why. 5. RINSE – Add 4 drops of water before rinsing well – dilute solution Part 3 – Heating of Water 1. Add 100 ml of water to a 250ml beaker. 2. Place the beaker on the hot plate and begin heating. 3. Bring the water to a boil. 4. Fill in your data table with your observations. 5. Hold a paper towel over the boiling water for a couple of minutes. 6. Place the paper towel on the lab bench and allow to cool. 7. Squeeze the paper towel and record your observations. 8. Determine if a chemical or physical change occurred and explain why. Part 4 – Hydrochloric acid with Magnesium Metal 1. Place a 3-4 drops of hydrochloric acid in one of the wells. 2. Add a small piece of magnesium metal to the acid. 3. Fill in your data table with your observations. 4. Determine if a chemical or physical change occurred and explain why. Physical and Chemical Changes Lab Data Table – Complete the following Data Table in your notebook Clues that Chemical Chemical or Drawing of what Change Occurred or Physical Change? you think the Did Not Occur molecules are doing Food Coloring to Water Mixing Two Solutions Boiling Water Adding Mg to HCl Analysis Questions 1. Sometimes a physical change produces the same clues as that normally happen with chemical changes. Did this happen in any of your procedures? Which ones? 2. What was the purpose of using the paper towel with the boiling water? How did it help you to decide whether a physical or chemical change was occurring? 3. Develop a definition for chemical change in your own words. 4. Develop a definition for physical change in your own words. 5. What is the difference between a chemical and physical change?