Document
advertisement

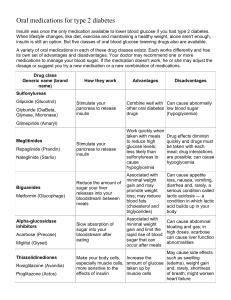
Presented by Orietta Girard and Marco Trigueros Internal Medicine Rotation JPH & RUSM Definitions of Diabetes Mellitus Epidemiology Classification of DM Etiology Criteria for the Diagnosis of DM Screening for DM Risk factors Pathogenesis Acute Complications of DM Chronic Complications of DM Approach to the Patient with DM Long Term Treatment/ Treatment Goals Nutritional Recommendations for Adults with Diabetes Management and ongoing care Questions/Answers References Diabetes mellitus (DM) refers to a group of common metabolic disorders that share the phenotype of hyperglycemia. Several distinct types of DM exist and are caused by a complex interaction of genetics and environmental factors. Factors contributing to hyperglycemia include: reduced insulin secretion, decreased glucose utilization, & increased glucose production. The metabolic dysregulation associated with DM causes secondary pathophysiologic changes in multiple organ systems. In the United States, DM is the leading cause of: end-stage renal disease (ESRD), nontraumatic lower extremity amputations, & adult blindness. It also predisposes to cardiovascular diseases. With an increasing incidence worldwide, DM will be a leading cause of morbidity and mortality for the foreseeable future. The worldwide prevalence of DM has risen dramatically over the past 20 yrs from: an estimated 30 million cases in 1985 to an estimated 177 million in 2000. Based on current trends, >360 million people will have diabetes by the year 2030. The prevalence of type 2 DM is rising much more rapidly than type 1 DM because of increasing obesity and reduced activity levels as countries become more industrialized. This is true in most countries, and 6 of the top 10 countries with the highest rates are in Asia. In the United States in 2005 statistics estimated that: 20.8 million persons, or 7% of the population, had diabetes (~30% of individuals with diabetes were undiagnosed). Approx. 1.5 million individuals (>20 yrs) were newly diagnosed Prevalence based on age group: 0.22% in those < 20 years 9.6% in those > 20 years. In individuals > 60 years, the prevalence of DM was 20.9%. The prevalence is similar in men and women throughout most age ranges but is slightly greater in men >60 years. Worldwide estimates project that in 2030 the greatest # of individuals with diabetes will be 45–64 years of age. DM is classified on the basis of the pathogenic process that leads to hyperglycemia, as opposed to earlier classification criteria such as age of onset or type of therapy. The two broad categories of DM are designated type 1 and type 2. Both types of diabetes are preceded by a phase of abnormal glucose homeostasis as the pathogenic processes progresses. Type 1 DM is the result of complete or near-total insulin deficiency. Type 2 DM is a heterogeneous group of disorders characterized by variable degrees of insulin resistance, impaired insulin secretion, and increased glucose production. Type 2 DM is preceded by a period of abnormal glucose homeostasis classified as impaired fasting glucose (IFG) or impaired glucose tolerance (IGT). Other etiologies for DM include: specific genetic defects in insulin secretion or action, metabolic abnormalities that impair insulin secretion, mitochondrial abnormalities, & a host of conditions that impair glucose tolerance Maturity onset diabetes of the young (MODY) is a subtype of DM characterized by autosomal dominant inheritance, early onset of hyperglycemia (usually <25 years), & impairment in insulin secretion. Genetic Defects of β cell Function characterized by mutation in: MODY 1 – HNF-4α MODY 2 - Glucokinase MODY 3 - HNF-1α MODY 4 – IPF-1 MODY 5 – HNF-1β MODY 6 - NeuroD1 Mitochondria DNA Subunits of ATP – sensitive K+ Channel Proinsulin or insulin conversion HNF – Hepatocyte Nuclear Transcription Factor IPF – Insulin Promoter Factor Mutations in the insulin receptor cause a group of rare disorders characterized by severe insulin resistance. DM can result from pancreatic exocrine disease when the majority of pancreatic islets are destroyed. Hormones that antagonize insulin action can also lead to DM. Thus, DM is often a feature of endocrinopathies. Viral infections have been implicated in pancreatic islet destruction but are an extremely rare cause of DM. A form of acute onset of type 1 diabetes, termed fulminant diabetes, has been noted in Japan and may be related to viral infection of islets. Genetic Defect of Insulin Action Endocrinopathies Type A insulin resistance Acromegaly Leprechaunism Cushing’s syndrome Rabson-Mendenhall syndrome Glucagonoma Lipodystrophy syndromes Pheochromocytoma Hyperthyroidism Somatostatinoma Aldosteronoma HNF – Hepatocyte Nuclear Transcription Factor IPF – Insulin Promoter Factor Gestational Diabetes Mellitus (GDM) Glucose intolerance may develop during pregnancy. Insulin resistance is related to the metabolic changes of late pregnancy, and the increased insulin requirements may lead to impaired glucose tolerance (IGT). GDM occurs in ~4% of pregnancies in the US; most women revert to normal glucose tolerance post-partum but have a substantial risk (30–60%) of developing DM later in life. Widespread use of the FPG as a screening test for type 2 DM is recommended because: (1) (2) a large number of individuals are asymptomatic & unaware, as DM may be present as much as 10 yrs before symptoms appear Early treatment of type 2 may favorably alter the history of DM. The ADA recommends screening all people > 45 years q 3 years & screening individuals at an earlier age if they are overweight (BMI) > 25 kg/m2, and have one additional risk factor for diabetes. In contrast, type 1 rarely has a long asymptomatic period of hyperglycemia Type 1 DM is the result of interactions of genetic, environmental, & immunologic factors that destroy the pancreatic β cells. Individuals with a genetic susceptibility (HLA-DR3/DR4 haplotypes, CTLA-4, IFIH-1, & PTPN-2 genes) have normal β cell mass at birth & begin to lose β cells 2dary to an autoimmune process triggered by an infectious or environmental stimulus. Most type 1 DM results from autoimmune β cell destruction but some individuals who have the clinical phenotype of type 1 DM lack immunologic markers (anti-GAD - glutamic acid decarboxylase) These individuals develop insulin deficiency by unknown nonimmune mechanisms, & are ketosis prone; many are African American or Asian descent. The rate of decline of β cell varies widely, with some patients progressing rapidly (months) to clinical diabetes & others evolving more slowly (years). The events that trigger the transition from glucose intolerance to frank diabetes are often associated with increased insulin requirements, as might occur during infections or puberty A "honeymoon" phase may ensue during which time glycemic control is achieved with modest doses of insulin or, rarely, insulin is not needed. Type 2 is characterized by impaired insulin resistance, abnormal insulin secretion, excessive hepatic glucose production, & abnormal fat metabolism. Insulin resistance (reduced glucose utilization in skeletal muscle) is present in many non-diabetic, 1st degree relatives of type 2 diabetics Environmental factors such as obesity(visceral/central), nutrition, & physical activity Type 2 DM has a strong genetic component. o 1 parent with type 2 DM increased risk of diabetes o 2 parents with type 2 DM increased risk by ≈ 40% o o The genes are incompletely identified, but current potential genes are: a variant of the transcription factor 7-like 2 gene peroxisome proliferators-activated receptor, inward rectifying K+ channel expressed in β cells, zinc transporter expressed in β cells, IRS, & calpain 10. Genetic mechanisms are predicted to be from alter insulin secretion In the early stages , glucose tolerance remains near-normal, despite insulin resistance, due to β cells compensation As insulin resistance & hyperinsulinemia progress, the pancreatic islets are unable to sustain the hyperinsulinemic state. IGT, then develops. A further decline in insulin secretion and an increase in hepatic glucose production lead to overt diabetes with fasting hyperglycemia. Ultimately, β cell failure may ensue. Diabetic ketoacidosis (DKA) & hyperglycemic hyperosmolar state (HHS) DKA formerly the hallmark of type 1 DM, also occurs in type 2 obese individuals (often of Hispanic or African American descent). HHS is primarily seen in type 2 DM. DKA & HHS are associated with absolute or relative insulin deficiency, volume depletion, and acid-base abnormalities. o Exist along a continuum of hyperglycemia, with or without ketosis. o Associated with serious complications if not promptly diagnosed & treated. The S&S of DKA usually develop over 24 h. DKA may be the initial symptom that leads to a diagnosis of type 1 DM, but more frequently it occurs in individuals with established diabetes DKA is initiated by inadequate levels of plasma insulin (relative or absolute deficiency) combined with counter-regulatory hormone excess (glucagon, catecholamines, cortisol, & growth hormone). The decreased ratio of insulin to glucagon: o o promotes gluconeogenesis, glycogenolysis, and ketone body formation in the liver, increases the substrate delivery from fat and muscle (FFAs, AAs) to the liver. Normally, these FFAs are converted to triglycerides or VLDL in the liver. In DKA, hyperglucagonemia alters hepatic metabolism to favor ketone body formation, through activation of the enzyme carnitine palmitoyltransferase I. Most commonly, DKA is precipitated by increased insulin requirements, as might occur during a concurrent illness. Failure to augment insulin therapy often compounds the problem. Therapeutic goals: alleviation of symptoms achievement of metabolic control prevention of acute and long term complications of diabetes Glycemic control Patient education Dietary modification Exercise Medications Set at the same goal for type 1 and type 2 diabetes preprandial blood glucose values of 90-130 mg/dL post-prandial values of <180mg/dL HbA1c of <7% (or as close as possible to normal) This degree of treatment has been associated with the lowest risk for long-term complications for both DM types Self monitoring of capillary blood glucose (SMBG) (especially for those with insulin); at least 3x’s a day. HbA1c every 2-3 months; or twice a year if well regulated Ketonuria monitoring with ketostix/acetest tablets if 1) persistent elevated glucose (>300mg/dL); 2) signs of impending DKA (N/V/abd. pain) 3) Febrile illness Reinforcement at every opportunity At routine check-ups Hospitalizations When complications take place Balanced diet to achieve adequate nutrition and maintain an ideal body weight. 1,000-1,200 kcal/d for women; 1,200-1,600 for men (if either overweight) Total caloric intake: 45-65% carbohydrates (adjust based on glycemic control) 10-30% protein < 30% total fat (<7% saturated fat & <300 mg/d cholest.) Those with high LDL, restrict fat intake to 25%, <200 chole) If diabetic nephropathy; further modifications on protein intake (.8 g/kg/d; down to .6 g/kg/d if it worsens). Grains and Starches (mostly carbohydrates) At base of the pyramid are bread, cereal, rice, and pasta. Made mostly of grains, such as wheat, rye, and oats. Starchy vegetables like potatoes, peas, and corn, along with dry beans such as black eyed peas and pinto beans. Starchy vegetables and beans have as much carbohydrate in one serving as a slice of bread. Choose 6-11 servings per day. Serving sizes are: 1 slice of bread 1/4 of a bagel (1 ounce) 1/2 an English muffin or pita bread 1-6 in tortilla 3/4 cup dry cereal 1/2 cup cooked cereal 1/2 cup potato, yam, peas, corn, or cooked beans 1 cup winter squash 1/3 cup of rice or pasta Vegetables Naturally low in fat and good choices as snacks/include in meals. Vegetables are full of vitamins, minerals and fiber. Spinach, chicory, sorrel, Swiss chard, broccoli, cabbage, bok choy, brussels sprouts, cauliflower, and kale, carrots, tomatoes, cucumbers, and lettuce. Choose at least 3-5 servings per day. A serving is: 1 cup raw 1/2 cup cooked Fruit Have plenty of carbohydrates, vitamins, minerals, and fiber. Blackberries, cantaloupe, strawberries, oranges, apples, bananas, peaches, pears, apricots, and grapes. Choose 2-4 servings per day A serving is: 1/2 cup canned fruit 1 small fresh fruit 2 Tbsp dried fruit 1 cup of melon or raspberries 1 1/4 cup of whole strawberries Milk and Dairy Contain protein and calcium as well as vitamins. Choose non-fat or low-fat dairy products for taste and nutrition without the saturated fat. Choose 2-3 servings per day A serving is: 1 cup non-fat or low-fat milk 1 cup of yogurt Meat and Meat Substitutes Beef, chicken, turkey, fish, eggs, tofu, dried beans, cheese, cottage cheese and peanut butter. Great sources of protein and many vitamins and minerals. Choose from lean meats, poultry and fish and cut all the visible fat off meat. Keep your portion sizes small; 3 oz is about the size of a deck of cards. Choose 4-6 oz per day divided between meals Equal to 1 oz of meat: 1/4 cup cottage cheese 1 egg 1 Tbsp peanut butter 1/2 cup tofu Fats & Sweets Potato chips, candy, cookies, cakes, crackers, and fried foods contain a lot of fat or sugar. Keep your servings small and save them for a special treat. Serving sizes include: 1/2 cup ice cream 1 small cupcake or muffin 2 small cookies Eat 45-60 grams of carbohydrate at a meal (more or less depending on how you manage diabetes). Foods that have carbohydrate: Starchy foods like bread, cereal, rice, and crackers fruit and juice milk and yogurt dried beans like pinto beans and soy products like veggie burgers starchy vegetables like potatoes and corn sweets and snack foods like sodas, juice drinks, cake, cookies, candy, and chips Non-starchy vegetables have a little bit of carbohydrate but in general are very low. How Much Carbohydrate is in Foods? Read food labels. Estimate how much carbohydrate in foods without labels. About 15 grams of carbohydrate in: 1 small piece of fresh fruit (4 oz) 1/2 cup of canned or frozen fruit 1 slice of bread (1 oz) or 1 (6 inch) tortilla 1/2 cup of oatmeal 1/3 cup of pasta or rice 4-6 crackers 1/2 English muffin or hamburger bun 1/2 cup of black beans or starchy vegetable 1/4 of a large baked potato (3 oz) 2/3 cup of plain fat-free yogurt or sweetened with sugar substitutes 2 small cookies 2 inch square brownie or cake without frosting 1/2 cup ice cream or sherbet 1 Tbsp syrup, jam, jelly, sugar or honey 2 Tbsp light syrup 6 chicken nuggets 1/2 cup of casserole 1 cup of soup 1/4 serving of a medium french fry Improves insulin sensitivity Reduces fasting and postprandial blood glucose Offers numerous metabolic, cardiovascular and psychological benefits in diabetic patients. Take a brisk walk (outside or inside on a treadmill) Go dancing Take a low-impact aerobics class Swim or do water aerobic exercises Try ice-skating or roller-skating Play tennis Stationary bicycle indoors Lift weights More effective when combined with exercise and diet Sulfonylureas: predispose to developing hypoglycemia in hospitalized patients not consuming routine diet. Metformin: with-held when iodinated radiocontrast dyes are used; renal dysfunction sepsis, CHF, or any condition that predisposes to lactic acidosis. Thiazolidinediones: not to be administered to patients with hepatic dysfunction, or CHF Glucosidase inhibitors: continued unless patient has GI illness. DPP-4 Inhibitors: predispose to developing hypoglycemia in conjunction with secretagogues/insulin. (watch dosage). Insulin: hypoglycemia!! Tight control needed! Add types of insulin and also there is new med called omeglide; Takes place within mins. Potent selective reversible inhibit. of dipeptidyl peptidase-4 Reduces degrad. of incretins & enhances actions of: glucagon-like peptide-1 GLP glucose insulinotropic polypeptide GIP. Improved β-cell function (increases insulin release) Suppression of glucagon secretion Improves glycemic control in monotherapy Provides additional efficacy in combination with other oral meds (metformin, sulfonylurea, thiazolidinedione). Absorbed rapidly PO and once daily dosing. Metabolized in vivo to form an active metabolite. Excreted primarily via the kidneys. Fasting and postprandial glucose concentrations are reduced Clinically meaningful reductions in HA1C. (increased glucose uptake by muscle & decreased hepatic glucose production). Low risk of hypoglycemia. Neutral effect on body weight Dose adjust. because of age, gender, or hepatic impair. not necessary. Insulin Type Onset (hr) Peak effect (hr) Duration Rapid Acting Lisro, aspart, glulisine 0.25-0.50 0.50-1.50 3-5 Regular 0.50-1.00 2-4 6-8 NPH 1-2 6-12 18-24 Lente 1-3 6-12 18-26 Ultralente 4-6 10-16 24-48 Glargine 4-6 None/flat effect 18 Detemir 3-4 Intermediate Acting Long Acting 20 Subcutaneous Delivery : Abdominal wall, thighs, buttocks, and arms May have continuous SC insulin infusion; using pump. Inhaled delivery: Recently approved; w/in 10 minutes before meal. (1mg = 2.5-3.0 units) Typical Regimen: Multiple daily insulin injections Long/Intermediate acting: 40-50% total daily dose as basal supply Remainder of doses givin wit rapid actin insulin divided across main meals. Initial dosages: 0.5-1.0 units/kg/d for average nonobese patient. Conservative doses with adjustments as BS is monitored. 1 unit insulin per 10-15g of carbohydrate. A set of individualized instructions meant to adjust dose of insulin in accordance with results of blood glucose levels, activity level and meals. Example; Regular Insulin is to be taken as per the following table: A. (2) units- BS <180 B. (4) units- BS 181-240 C. (6) units- BS 241-320 D. units- BS 321-400 E. units- BS >401 Insulin ½ hour before a meal may be suggested Create a correct and detailed record of test timings, test result and amount (especially if glucose levels are controlled). Check BS at set times a day; ie. 1 hour post-prandially q meal. Long and short acting insulin may be co-administered to mimic natural insulin release and adjusted as sliding scale is modified. DKA glucose >250 mg/dL and pH <7.30 or bicarb <15mEq/L and ketonuria/ketonemia Hyperosmolar nonketotic state marked hyperglycmia >400mg/dL elevated osmolality >315 mOsm/Kg Hypoglycemia induced by sulfonylurea resulting in coma, seizures or altered mentation Newly Diagnosed type 1 DM and Gestational DM even those without ketoacidosis Newly Diagnosed type 2 DM Even in absence of ketoacidosis or hyperosmolar syndrome An 89-year-old woman is evaluated in a nursing home. She has had diabetes for more than 15 years; she was treated with a sulfonylurea for 1 year, but subsequently required insulin therapy. She has recently been experiencing labile control, with blood glucose levels fluctuating widely between 50 mg/dL (2.78 mmol/L) and more than 300 mg/dL (16.65 mmol/L). She takes 70/30 NPH/Regular insulin, 22 units AM and 18 units PM. During a recent episode of gastroenteritis, her morning insulin was withheld because of concern that she would consume few calories that day. At 4 PM that day, her glucose level was 513 mg/dl (28.47 mmol/L). Her medical history is notable for stroke, coronary artery disease, and colon cancer. Examination shows a thin woman (BMI 21) who looks her stated age. Dipstick urinalysis shows glucose and ketones. Which of the following is the appropriate categorization of this patient's diabetes? A Type 1 diabetes mellitus B Type 2 diabetes mellitus C Secondary diabetes D Latent autoimmune diabetes of adulthood (LADA) The answer is D A minority of patients previously diagnosed with type 2 DM develop insulin requirements later in life and exhibit the labile glycemic tendencies and many of the autoimmune markers (ex.: anti-GAD (Anti-Glutamic-AcidDecarboxylase) 65 antibodies) that are detected type 1 DM. This form of diabetes, called latent autoimmune diabetes of adulthood (LADA), also involves loss of beta cell function which progresses & ultimately becomes severe insulin deficiency. Its cardinal manifestation is labile glycemic control in a lean, older patient, which fits the description of this patient. These patients often have a prior history of good glycemic control with oral agents alone. It is important to identify LADA because affected patients quickly become refractory to oral agents and are just as insulin-dependent and become as ketosis prone as the typical patient with Type 1 DM. Accordingly, during times of illness, basal insulin must be continued, with additional doses of short-acting insulin provided as needed to prevent marked hyperglycemia. A 50-year-old female is 5 ft 7 in. tall and weighs 165 lb. There is a family history of diabetes mellitus. Fasting blood glucose is 150 mg/dl on two occasions. She is asymptomatic, and physical exam shows no abnormalities. The treatment of choice is A) Observation B) Medical nutrition therapy C) Insulin D) Oral hypoglycemic agent The correct answer is b The classification of diabetes mellitus has changed to emphasize the process that leads to hyperglycemia. Type 2 DM is a group of heterogeneous disorders characterized by insulin resistance, impaired secretion of insulin, and increased glucose production. Medical nutrition therapy is a term now used to describe the best possible coordination of calorie intake, weight loss, and exercise. It emphasizes modification of risk factors for hypertension & hyperlipidemia, not just weight loss and calorie restriction. In this type 2 patient, medical nutrition therapy that includes dietary modification, weight loss, and exercise is the first intervention. Blood glucose control should be reevaluated after 3 to 4 weeks. If target blood sugar is not met, pharmacotherapy should be initiated. A 32 years old primigravid woman at 35 weeks' gestation comes to the physician for a prenatal visit. At 26 weeks, she failed her 50-g, 1-hour oral glucose-loading test. She also failed her follow-up 100-g, 3-hour oral glucose tolerance test, with a normal fasting glucose, but abnormal 1, 2, and 3-hour values. Over the past several weeks, she has maintained good control of her fasting and 2-hour postprandial glucose levels by adhering to the diet recommendations of her physician. She asks the physician what effect her type of diabetes can have on her or her fetus. Which of the following is the most appropriate response? A. B. C. D. E. Gestational diabetes is associated with fetal anomalies Gestational diabetes is associated with intrauterine growth restriction Gestational diabetes is associated with fetuses weighing 4000 g or more Gestational diabetes is not associated with future diabetes Gestational diabetes with normal fasting glucose is associated with stillbirth The correct answer is C. Patients with GDM & normal fasting glucose levels have two major risks. o Fetal macrosomia o Eventual development of overt diabetes (50% increase risk in next 20 yrs) Choice A is incorrect. However, patients with overt diabetes do have an increased risk of fetal anomalies. Choice B is incorrect. GDM is associated with macrosomia. Choice D is incorrect. GDM is not associated with future diabetes. Choice E is incorrect. Abnormal fasting glucose is associated with stillbirth in both GDM & overt diabetes www.Access Medicine.com Harrison’s Online > part 15th : Endocrinology & Metabolism > Section 1: Endocrinology > Chapter 338, Diabetes Mellitus Daniel H. Cooper MD et all. The Washington Manual of Medical Therapeutics, 32th edition, pp 600-620. Carolyn F. Deacon and Jens J. Holst. Advances in Therapy. Springer Healthcare Communications; Volume 26, Number 5 / May, 2009, pp488499. www.diabetes.org ADA>Food & Fitness