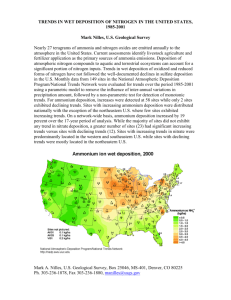
E.2-Environmental-chemistry-acid
advertisement
E2 acid deposition • State what is meant by the term acid deposition and outline its origins. • Discuss the environmental effects of acid deposition and possible methods to counteract them. acid deposition • Acid deposition refers to the process by which acidic particles, gases and precipitation leave the atmosphere as they are brought back down to earth e.g. on the ground, on trees, buildings, inside plants or animals (e.g. your lungs). • There are two types of acid deposition: wet deposition: acid rain (pH less than 5.6), fog and snow dry deposition: acidic gases such as SO2 and salts (when acidic gas reacts with an alkali) • All rain water is naturally acidic owing to the presence of dissolved carbon dioxide, which reacts with water to form carbonic acid: • CO2(g)+H2O(l)⇄H2CO3 • Sulfur dioxide dissolves in water to form sulfurous acid H2SO3 • H2O(l)+SO2 (g)→H2SO3 • Sulfuric acid H2SO4 (aq) is formed when the sulfur dioxide is oxidized to sulfur trioxide which then dissolves in water. acid deposition NOx: formed as a result of high temperatures in internal combustion engines, i.e. cars and jet engines. 1. production of nitrogen oxides: N2 (g) + O2 (g) 2NO (g) 2NO (g) + O2 (g) 2NO2 (g) 2. the following equations show two ways in which nitric acid is formed in the atmosphere 2 NO2 (g) + H2O (l) HNO3 (aq) + HNO2 (aq) (=nitrous acid) or 4 NO2 (g) + 2 H2O (l) + O2 (g) 4 HNO3 (aq) acid deposition SOx: from burning of coal which contains sulphur/smelting plants 1. production of oxides: S (g) + O2 (g) SO2 (g) 2SO2 (g) + O2 (g) 2SO3 (g) 2. formation of atmospheric sulphuric acid and sulfurous acid SO3 (g) + H2O (l) SO2 (g) + H2O (l) H2SO4 (aq) H2SO3(aq) acid deposition humans buildings irritation of the mucus membranes and lung tissue when breathing in fine droplets of acid rain; increase in risk of respiratory illnesses like asthma and bronchitis; acidic water also dissolves and leaches poisonous ions like Al3+ (linked with Alzheimer disease) and Pb2+. corrosion of materials such as marble and dolomite (CaCO3.MgCO3): equation: CaCO3 (s) + H2SO4 (aq) CaSO4(s) + H2O(l) + CO2 (g) faster corrosion of iron and steel structures in buildings or bridges. acid deposition aquatic life vegetation increased levels of aluminum ions dissolved from the soil kills fish as it effects the function of the gills; a lot of fish, algae, insect larvae even plankton cannot survive in water below a certain pH. increased soil acidity leaches important nutrients (e.g. Ca2+/K+/ Mg2+) out of the top soil Mg2+is necessary to make chlorophyll so removal of this ion results in lowering rate of photosynthesis and reducing growth of plants and crop yields. Increased concentration of Al3+ which damages roots stunted growth, thinning of tree tops, yellowing and loss of leaves. Effects on water • The nitric acid present in acid rain poses a particular problem as the nitrates present can lead to eutrophication. The nitrate ions promote excessive plant growth causing plants to need more oxygen to be available in the water supply, which can in turn lead to other plant deaths. Control strategies • Acid deposition can be controlled by reducing the level of emission of nitrogen and sulfur oxide. The possible method is to switch to alternative energy sources, such as wind, solar or tidal energy, or to reduce the demand for fossil fuels by using more public transport or more efficient energy transfer systems. The role of ammonia in acid deposition • Ammonia is present in the atmosphere from both natural and synthetic sources. It is produced naturally by animal livestock and by the action of certain bacteria and also from artificial fertilizers. • NH3+HNO3→NH4NO3 • 2NH3+H2SO4→(NH4)2SO4 acid deposition • cutting down emissions of nitrogen oxides by using catalytic converters; thermal exhaust systems; • use of low sulphur fuels; use of these fuels still release sulphur dioxide • removal of sulphur oxides from exhaust fumes (Flue Gas Desulphurization); • use of alternative sources of energy which do not involve fossil fuels and the production of very high temperatures. • dealing with acid rain itself: neutralization of lakes using calcium carbonate or calcium oxide, e.g. CaO + H2SO4 (aq) CaSO4 (s) + H2O (l)