Kinetic Molecular Theory and the Gas Laws
advertisement

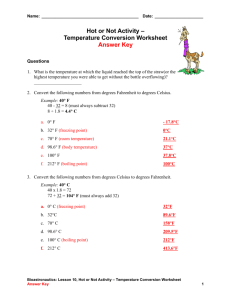
Kinetic Molecular Theory and the Gas Laws Phases of Matter, Kinetic Molecular Theory, and Temperature Gas Laws Phase Changes Phases of Matter There are four phases of matter: 1. Solid 2. Liquid 3. Gas 4. Plasma The state of matter depends on the motion of the molecules that make it up. Solids Solids are objects that have definite shapes and volumes. The atoms or molecules are tightly packed, so the solid keeps its shape. The arrangement of particles in a solid are in a regular, repeating pattern called a crystal. Microscopic picture of a solid. Liquids The particles in a liquid are close together, but are able to move around more freely than in a solid. Liquids have no definite shape and take on the shape of the container that they are in. Microscopic picture of a liquid. Gases A gas does not have a definite shape or volume. The particles of a gas have much more energy than either solids or liquids and can move around freely. Microscopic picture of a gas. Plasma Plasma is a gas-like mixture of positively and negatively charged particles. Plasma is the most commonly found element in the universe, making up 99% of all matter. It is found in stars, such as the sun, and in fluorescent lighting. Plasma occurs when temperatures are high enough to cause particles to collide violently and be ripped apart into charged particles. Kinetic Molecular Theory of Matter All matter is made of particles that are in constant motion. The more energy the particles have, the more freely they move around. This freedom that molecules have is the determining factor for their state of matter. Therefore, solids have the least amount of energy. Liquids come next followed by gases. Finally, plasma has the most energy of any state of matter. Temperature Temperature is a measure of the amount of the average kinetic energy of the particles in matter. The more kinetic energy the particles have, the higher the temperature. The temperature of particles are usually recorded in one of three ways: 1. Fahrenheit (ºF) 2. Celsius (ºC) 3. Kelvin (K) Do you remember which is the standard unit???? Fahrenheit Developed by Daniel Gabriel Fahrenheit, who is best known for inventing the alcohol thermometer and mercury thermometer in the early 1700’s. It is based on 32º for the freezing point of water and 212º for the boiling point of water. The interval between the freezing and boiling points are divided into 180 parts. The conversion to Celsius is: ºF = (9/5 ºC) + 32 Celsius Scale developed by Anders Celsius in the early to mid-1700’s, working from the invention of Fahrenheit's thermometers. The Celsius scale is based on 0º for the freezing point of water and 100º as the boiling point. The interval between the freezing and boiling points are divided into 100 parts. The conversion to Fahrenheit is: ºC= (5/9)(ºF-32) The conversion to Kelvin is: K=ºC +273 Kelvin Developed by William Thompson Kelvin in 1848, Kelvin is a temperature scale having an absolute zero below which temperatures do not exist. At 0K, all molecules cease any type of motion (as in the temperature of outer space). It corresponds to a temperature of -273° on the Celsius temperature scale. The Kelvin degree is the same size as the Celsius degree, so the freezing point of water is at 273K and the boiling point is at 373K. Return to Home Page The Behavior of Gases The behavior of gases can be explained by the way their particles interact with each other and the environment around them. The particles are constantly colliding with one another and other objects. Since the molecules have mass, there is a certain amount of pressure being applied. As the volume of the gas and/or the temperature of the gas change, so does its behavior. Gas Laws The result of a force distributed over an area. SI unit for pressure = pascal (Pa) = N/m2 (one kilopascal = kPa= 1000 Pa) Factors that Affect Pressure of an Enclosed Gas Temperature Volume Number of Particles Temperature Raising the temperature of a gas will increase its pressure if the volume of the gas and the number of particles are constant Volume Reducing the volume of a gas increase its pressure if the temperature of the gas and the number of particles are constant. Number of Particles Increasing the number of particles will increase the pressure of a gas if the temperature and the volume are constant. Boyle’s Law Boyle’s Law shows the relationship between the volume and pressure of a gas: The volume of a fixed amount of gas varies inversely with the pressure on the gas. If the pressure increases, the volume decreases; if the pressure decreases, then the volume increases. P1V1=P2V2 Boyle’s Law From Physical Science, Merrill, 1993 Boyle’s Law A Graph of Boyle’s Law Charles’s Law Charles’s Law shows the relationship between the temperature and volume of a gas: The volume of a fixed amount of gas varies directly with the temperature of the gas. If the temperature increases, the volume increases; if the temperature decreases, then the volume decreases. V1 = V2 T1 T2 A Graph of Charles’s Law Return to Home Page The Combined Gas Law The relationships described by Boyle’s law and Charles’ law can be described as a single law. The combined gas law describes the relationship among the temperature, volume, and pressure of a gas when the number of particles is constant. P1V1 P2V2 T1 T2 Phase Changes A reversible physical change that occurs when a substance changes from one state of matter to another. The temperature of a substance doesn’t change during a phase change. Energy is either absorbed or released during a phase change. Heat of fusion = energy a substance must absorb in order to change from a solid to a liquid. Six Common Phase Changes Melting- temperature at which a substance changes from solid to liquid. 2. Freezing – temperature at which a substance changes from a liquid to a solid. 3. Evaporation – substance changes from a liquid to a gas. (Heat of Vaporizationenergy a substance must absorb in order to change from a liquid to a gas.) 1. 4. Condensation- substance changes from a gas or vapor to a liquid. 5. Sublimation – substance changes from a solid to a gas or vapor without changing to a liquid first (endothermic) 6. Deposition – substance changes directly into a solid without first changing to a liquid (exothermic) Return to Home Page