Osmosis An Important Type of Diffusion
advertisement
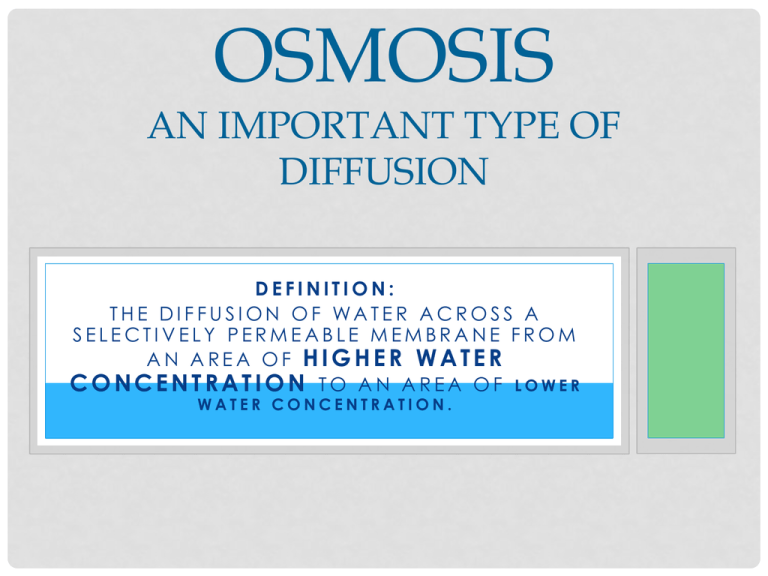
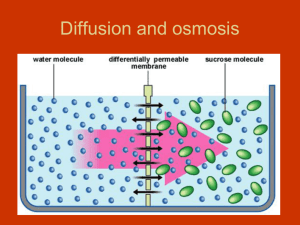
OSMOSIS AN IMPORTANT TYPE OF DIFFUSION DEFINITION: THE DIFFUSION OF WATER ACROSS A SELECTIVELY PERMEABLE MEMBRANE FROM AN AREA OF HIGHER WATER CONCENTRATION TO AN AREA OF LOWER WATER CONCENTRATION. WATER…WOW! • Water is vital to life • Plants and animals use water to carry out essential life processes • Water particles are small enough to travel across the cell membrane in both directions • If the water concentration is equal on both sides, the cell maintains its size. WHAT HAPPENS WHEN THERE IS AN IMBALANCE? • If there is more water either inside or outside the cell, the direction in which water moves across the cell membrane adjusts to this imbalance • More water will move in one direction than the other Definition: SOLUTE A substance that is dissolved in another substance – ie. Sugar and salt • Water moves by osmosis from an area with higher water (or lower solute) concentration to an area with lower water (or higher solute) concentration. • Osmosis continues until the concentration of water is equal on both sides of the membrane. • When concentrations are equal, osmosis ends and water continues to pass through the membrane in both directions at an equal rate. OSMOSIS CELLS IN SOLUTION • Sugars, salts and proteins are common solutes in cells • Cells need to maintain solute concentrations at certain levels to stay alive and healthy • The movement of water into and out of the cell determines solute concentration Environment A • Concentration of water molecules outside of the cell is EQUAL to concentration of water molecules inside the cell. • Movement of water is continuous • Shape and size of cell do not change Environment B • Concentration of water molecules GREATER OUTSIDE than inside the cell. • More water molecules move INTO the cell than out • Cell INCREASES in size • If too much water enters the cell it may burst and die Environment C • Concentration of water molecules GREATER INSIDE the cell than outside • More water molecules move OUT of the cell • Cell DECREASES in size • If too much water leaves the cell, it may die. TURGOR PRESSURE: OSMOSIS IN ACTION • Plant cells have a large, central vacuole which is filled with water • This vacuole takes up much of the cell’s interior space • Water absorbed by the plants roots is stored in vacuoles • When a cell needs water for cellular processes, water moves from the vacuole to the parts of the cell where it is needed • This movement causes a decrease in the water in the cells cytoplasm, and an increase in the concentration of solutes • When solute concentration inside the plant cell becomes high, water moves into the cell by osmosis • When the vacuole and cytoplasm fill with water, this exerts pressure against the cell wall, and causes the cell to swell. • This outward pressure on the cell wall is called TURGOR PRESSURE. • When cells in the plant’s stem and leaves take in water, they become “turgid” – they stiffen and stay upright (a) • When cells in a plant’s stem and leaves lose water, the cells become less turgid, and the plant wilts (b) • Problems occur when plants lose too much water. • If the concentration of solutes increases in the soil – this lowers the concentration of water in the soil compared to the concentration of water in the plant roots • This causes water to move by osmosis from the plants roots to the soil • Why is this important to understand in the farming industry when dealing with fertilizers? EXIT SLIP • Salting roads in the winter often results in a build up of salt, which causes the plant life beside the roads to die. Why are salty soil conditions not good for plants? Explain.