Protein
advertisement

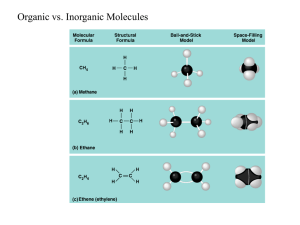
Are you what you eat? 1. The important Characteristics of Carbon Forms 4 covalent bonds Forms double and triple bonds Forms long chains and rings Can bind with many other elements Even electron distribution (nonpolar molecules) 2. Macromolecules, Monomers and Polymers (Hint: think of the meaning of the prefixes) What do these words mean? Polygons Polyester Polygamy 2. Macromolecules, Monomers and Polymers Polymer – Smaller organic molecules join into long chains. Monomer – the individual unit that builds up polymers Macromolecules – Very large molecules 3. Dehydration synthesis and Hydrolysis These two terms refer to the processes that forms monomers and polymers: Dehydration synthesis – A reaction that removes molecules of water to form polymers from monomers Hydrolysis – The reaction that adds water to polymers to separate them to their individual monomers. (http://nhscience.lonestar.edu/biol/dehydrat/dehydrat.html or http://www.youtube.com/watch?v=UyDnnD3fMaU ) Isomers Molecules that have the same formula, but different structures. Examples: Glucose and Fructose 4. What are the big four? Three out of the 4 types of biochemical macromolecules can be found on food nutrition labels… Look at the label to the left. 3 of the 4 macromolecules can be found in foods. 1____________________ (0 grams in this product) (13 grams in this product) 2____________________ (9 grams in this product) 3____________________ 4. What are the big four? Fats (we call them lipids) Carbohydrates Proteins Nucleic acids (DNA and RNA) When studying these biochemical molecules, we are interested in finding out….. what they do for living things. what they generally look like. what their monomers are. and how they may help the body gain energy to sustain life. SO, LETS GET STARTED! Great website for reference… http://biomodel.uah.es/en/model3/index.htm 5. Carbohydrates Molecules that form from atoms in 1C:2H:1O ratio Monomers: Monosaccharides (simple sugars) Monosaccharides are usually sweet, white powdery substances (such as fructose, glucose) that form rings of carbon atoms. Monosaccharides in general serve as direct, quick sources of energy for living organisms during cellular respiration, they are building blocks of many polymers Important monosaccharides: Glucose Fructose Disaccharides – two monosaccharide molecules combine by dehydration synthesis to form disaccharides Important disaccharides: Lactose – found in milk sugar Sucrose – table sugar Polysaccharides – many (tens to hundreds) units of monosaccharides combine by dehydration synthesis Polysaccharides also separate to monosaccharides by hydrolysis while taking in water. Important polysaccharides: Starch – made up of many glucose units, it is an important storage polysaccharide that is found in plant roots and other tissues. It stores monosaccharides that can be broken down later to release useful energy during cellular respiration – ONLY IN PLANTS Glycogen – also made up of many glucose units, it is an important storage polysaccharide in the liver and animal muscles. It can also be broken down to monomers to release energy during cellular respiration. ONLY IN ANIMALS Cellulose – also made up of many glucose units. However, in this case the molecule is not easily broken down to its monomers. It is important for providing a rigid structure in plant cell walls. Chitin – made up of some nitrogen containing monosaccharides. It is an important polysaccharide that provide the solid structure of arthropods and fungi. 6. Lipids a diverse group of molecules that are nonpolar and generally do not dissolve in water They mostly contain carbon, hydrogen, very few oxygen atoms, but some also have phosphorous. There are three distinct groups of lipids: Simple lipids Phospholipids Sterols 6A. Simple Lipids Very large molecules that form from 2 different kinds of monomers by dehydration synthesis: 3 Fatty acids – are long chains of carbon with oxygen at the end (can be saturated and unsaturated) 1 Glycerol – smaller 3-carbon compound. Simple lipids are important as storage materials in all living things. They can store twice as many calories as polysaccharides can. Oils (mostly from plants) contain more unsaturated fatty acids, while fats (animals) contain more saturated fatty acids. Simple lipids also dissolve vitamins http://biomodel.uah.es/en/model3/index.htm 6B. Phospholipids Phospholipids – phosphate containing lipids. Their monomers: 1 glycerol + 2 fatty acids (saturated or unsaturated) + phosphate. These monomers combine by dehydration synthesis Phospholipids have both polar and nonpolar sections. As a result, they are able to dissolve in both type of solvents as well. They are important for living things because they form the borders of all cells (cell membranes) and also participate in forming many cell organelles. 6C. STEROLS Sterols are a highly nonpolar (hydrophobic) group of molecules. They occur naturally in plants, animals, and fungi, with the most familiar type of animal sterol being cholesterol. Cholesterol is vital to cellular function, and a precursor to fat-soluble vitamins and steroid hormones. 7. Proteins Protein- Polymer constructed from amino acid monomers. Only 20 amino acids, but make 1,000s of proteins Some are 100 a.a. in length; some are thousands 3-D Protein 7A. Protein Functions Each of our 1,000s of proteins has a unique 3-D shape that corresponds to a specific function: Defensive proteins Antibodies in your immune system Signal proteins Hormones and other messengers Hemoglobin Delivers 02 to working muscles Transport proteins Move sugar molecules into cells for energy (insulin) Storage proteins Ovalbumin (found in egg white) used as a source of amino acid for developing embryos Most important roles is as enzymes Chemical catalysts that speed and regulate virtually all chemical reactions in cells Example, lactase 7B. Amino Acid structure Proteins diversity is based on differing arrangements of 20 amino acids. Amino acids all have an amino group and a carboxyl group. R group is the variable part of the amino acid; determine the specific properties of the 20 amino acids. Two main types: Hydrophobic Example: Leucine R group is nonpolar and hydrophobic Hydrophilic Polar and charged a.a.’s help proteins dissolve in aqueous solutions inside cells. Example: Serine R group is a hydroxl group 7C. Amino Acid Dehydration Cells join amino acids together in a dehydration reaction: Links the carboxyl group of one amino acid to the amino group of the next amino acid as a water molecule is removed. Form a covalent linkage called a peptide bond making a polypeptide.