ESSENTIAL QUESTION: HOW DO WE RECOGNIZE
advertisement

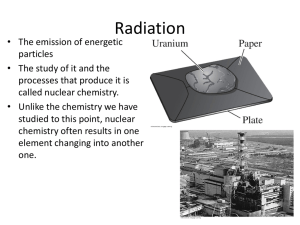
Radioactivity is invisible. We are also unable to hear, taste, touch, or smell it. Yet we are able to detect and measure radioactivity with certain scientific instruments such as a Geiger counter. This device allows us to use radioactivity safely because it lets us know when even tiny amounts of radiation are present. The rock layers exposed in the Grand Canyon represent intervals of time stretching back hundreds of millions of years. Radioactivity is a scientific process. One of the most important ideas in all of Earth science is the concept of geologic time. Because Earth is covered in rocks, radioactivity can be used to determine the age of the Earth. Radioactive decay of unstable isotopes contained in rock layers can be used to determine the absolute age of the rocks. Radioactive decay occurs when the original parent isotope is converted to a daughter atom. Radioactive decay is measured in half-lives, which is the length of time taken for one-half of the radioactive material to decay. The half-life of an isotope is the time taken for half of the original parent isotopes to decrease to daughter atoms. Are they the same thing? Radioactivity is the name given to the processes where an unstable nucleus of an atom changes into a different nucleus. Particles such as alpha, beta and gamma are given off during the change. The disintegration process of these particles is known as nuclear radiation. Radiation is technically any form of energy that can travel through space in the form of waves. So, light, microwaves and radio/TV broadcasting waves are all technically forms of radiation, but are not produced by radioactivity. What do the following materials have to do with each other? •Rock samples •Fiestaware pottery dishes •Bananas •Coleman lantern mantle •TV set •Phosphate fertilizer •Brick DETECTION OF RADIOACTIVITY Yes. Most radioactive substances enter our bodies as part of food, water or air. Our bodies use the radioactive as well as the nonradioa Yes. Most radioactive substances enter our bodies as part of food, water or air. Our bodies use radioactive as well as the nonradioactive forms of vital nutrients, such as iodine and sodium. Radioactivity can be found at every step of the food chain. It is even in our drinking water. Yes. Another type of natural radiation is cosmic radiation from the Sun and outer space. Because Earth’s atmosphere absorbs some of this radiation, locations at higher altitudes receive more exposure. In Ohio, for example, the average resident receives a dose of about 40 millirem in one year from cosmic radiation. In Colorado, it is about 180 millirem in one year. Generally, for each 100-foot increase in altitude, there is an increased dose of one millirem per year. Flying in an airplane increases our exposure to cosmic radiation. A coast-to-coast round trip gives us a dose of about four millirem. Yes. Radiation in soil and rocks contributes about 60 millirem in one year to our exposure. In Colorado, it is about 105 millirem per year. In India, radioactivity from soil and rocks can be 3,000 millirem per year, and at a beach in Brazil, it is over 5 millirem in a single hour -- but only a few residents who use that beach receive doses in excess of 500 millirem per year. Yes. If you live in a wood house, the natural radioactivity in the building materials gives you a dose of 30 to 50 millirem per year. In a brick house, it is 50 to 100 millirem per year. And, if your home is so tightly sealed that there is little ventilation, natural radioactive gases (radon) can be trapped for a longer period of time and thus increase your dose. Even some granite countertops (particularly reds and purples) may contain some radioactivity. Yes, it’s true we can’t escape from radioactivity in our everyday environment. Each person with whom we spend eight hours a day gives us a dose of about 0.1 millirem in a year. Using a gas stove increases the dose by about 2 millirem per year because of radioactive materials in the natural gas. A person who smokes two packs of cigarettes a day receives a radiation dose of about 1,300 millrem per year. This is because polonium (a radioactive element) is part of the smoke and when inhaled, it gets trapped in the lungs. RADIATION REALLY IS EVERYWHERE. Every day we are exposed to a constant stream of radiation from the Sun and outer space. Radioactivity is in the ground, air, the buildings we live in, the food we eat, the water we drink, and the products we use. Remember that lead barrier apron you wear when you get x-rays done at the dentist’s office? The average person in the United States receives a dose of about 360 millirem per year from natural sources of radioactivity as well as from typical medical radiation exposures. Is it true that we can’t live without radioactivity? Yes, it’s true. Our bodies are radioactive. It’s a simple fact of nature. But there is no cause for alarm. These very small but detectable levels of radioactivity are natural . . . as natural as life itself. To put this into perspective… although one’s risk increases with increased exposure, no harmful effects have ever been observed at levels below 5,000 millirem. In fact, effects seen when humans are exposed to 100,000 millirem over a short time period are temporary and can be reversible. It takes a short-term dose of more than 500,000 millirem to cause a fatality so the level of radiation you receive and the rate you receive it in is important. What happened in Japan? People in Japan that lived within 12 miles of the March 2011 Fukushima earthquake/nuclear meltdown disaster area were exposed to … What happened in Japan? What happened in Japan? What happened in Japan? In March 2011, Japanese officials announced that "radioactive iodine-131 exceeding safety limits for infants had been detected at 18 water-purification plants in Tokyo and five other prefectures". As of July 2011, the Japanese government was still unable to control the spread of radioactive material into the nation’s food. Radioactive material was detected in a range of produce, including spinach, tea leaves, milk, fish and beef, up to 200 miles from the nuclear plant. Inside the 12-mile evacuation zone around the plant, all farming has been abandoned. Nuclear radiation leak in Japan Half-life of… Radioactive iodine-131 8.0197 days The fate of Japan… As of February 2012, the crippled Fukushima nuclear plant is still leaking radiation and areas surrounding it could remain uninhabitable for decades due to high radiation. It could take “more than 20 years before residents could safely return to areas with current radiation readings of 200 mSv/h (millisieverts) per year, and a decade for areas at 100 mSv/h per year”. So how much is a millirem? A rem is a large dose of radiation, so the millirem (mrem), which is one thousandth (1/1000th) of a rem, is used for the dosages of radiation received from medical x-rays or for other medical diagnostic purposes. An acute whole-body dose of under 50 rem will produce nothing other than blood changes. 50 to 200 rem may cause illness but will rarely be fatal. Doses of 200 to 1,000 rem will probably cause serious. Doses of more than 1,000 rems are almost invariably fatal. 1 Sv = 100 rem 0.000010 Sv = 0.010 mSv So, should we be concerned that there is radioactivity in our everyday environment? No, not unless you get exposed to too much over a short period of time.