Chemical reactions and Enzymes
advertisement

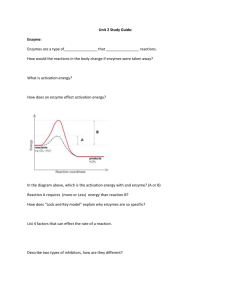
• Have you ever sat around a campfire or watched flames flicker in a fireplace? The burning of wood is a chemical reaction—a process that changes one set of chemicals into another set of chemicals. A chemical reaction always involves changes in chemical bonds that join atoms in compounds. The elements or compounds that enter into a chemical reaction are called reactants. The elements or compounds produced by a chemical reaction are called products. As wood burns, molecules of cellulose are broken down and combine with oxygen to form carbon dioxide and water vapor, and energy is released. • 1. What are the reactants when wood burns? • 2. What are the products when wood burns? • 3. What kinds of energy are given off when wood burns? • 4. Wood doesn’t burn all by itself. What must you do to start a fire? What does this mean in terms of energy? • 5. Once the fire gets started, it keeps burning. Why don’t you need to keep restarting the fire? Concept Map of Organic Compounds Section 2-3 *Fill in the Blanks* Carbon Compounds include that consist of that consist of that consist of that consist of which contain which contain which contain which contain Concept Map of Organic Compounds Section 2-3 *Answer Key* Carbon Compounds include Carbohydrates Lipids Nucleic acids Proteins that consist of that consist of that consist of that consist of Sugars and starches Fats and oils Nucleotides Amino Acids which contain which contain Carbon, hydrogen, oxygen Carbon, hydrogen, oxygen which contain which contain Carbon,hydrogen, oxygen, nitrogen, phosphorus Carbon, hydrogen,oxygen, nitrogen, Chemical reactions and Enzymes Chapter 2.4 Chemical Reactions • Chemical reaction – a process that changes one set of chemicals into another set of chemicals ex. Iron and oxygen combine to form iron oxide or rust • Always involve changes in the chemical bonds that join atoms in compounds Chemical Reactions • The elements or compounds that enter into a chemical reaction are known as reactants • The elements or compounds produced by a chemical reaction are known as products Energy in reactions • Bonds are the storage place of energy in molecules / compounds – Break a bond release energy – Make a bond store energy Energy in Reactions • Chemical reactions that release energy often occur spontaneously • Chemical reactions that absorb energy – will not occur without a source of energy • All living things carry out energy-needing reactions – All have their own source of energy • Sun • Chemicals • food Energy in Reactions Energy-Absorbing Reaction Products Activation energy Reactants Energy-Releasing Reaction Energy in Reactions Energy-Absorbing Reaction Energy-Releasing Reaction Activation energy Products Activation energy Reactants Reactants Products Activation energy energy needed to get a reaction started • Required whether energy is released or absorbed. Enzymes • Catalyst – A substance that speeds up the rate of a chemical reaction – Lowers the reaction’s activation energy • Biological catalysts = enzymes Enzymes • Living cells use enzymes to speed up virtually every important chemical reaction that takes place inside cells • Lowers the activation energy required for the reaction to occur thereby increasing the rate in which the reaction takes place. Reaction pathway without enzyme Reactants Reaction pathway with enzyme Activation energy without enzyme Activation energy with enzyme Products Review • What is the difference between the reactant and a product? Give an example • How is energy released and stored within a chemical compound? • Does both energy absorbing and energy releasing reactions require activation energy? What is activation energy? • What is one benefit to a catalyst? Enzyme Example • Cells release carbon dioxide into the blood – Carbon dioxide will not dissolve in blood • Reacts with water to become carbonic acid which is soluble in blood – When carbonic acid gets to lungs, converts back to carbon dioxide Enzyme Example • Left by itself, reaction probably would not take place fast enough to remove carbon dioxide from our body – We would die • Enzyme (carbonic anhydrase) – Speeds up reaction 10 million times Enzyme Specificity • Enzymes are very specific catalyze only one chemical reaction • Part of the name of the enzyme is derived from the reaction it catalyzes – Carbonic anhydrase removes water from carbonic acid Enzyme Action • If enzymes are not present, reactants must randomly collide with one another with enough energy to make products • Enzymes make process faster and easier by getting involved in reactions Enzyme action • Substrates the reactants of an enzyme-catalyzed reactions • Bind to enzyme at active site – Form enzyme-substrate complex – Very specific – like a lock & key Enzyme action • Once complex is formed, enzyme does its job of making products of reaction – Products are released – Enzymes go to bind with new substrates (they are recycled) – P. 52 Figure 2-21 Enzyme Action Enzyme action Enzyme action is very sensitive to environmental conditions pH temperature Enzymes play an essential role in regulating chemical pathways making materials cells need releasing energy transferring information