File - Class work Science JC and LC
advertisement

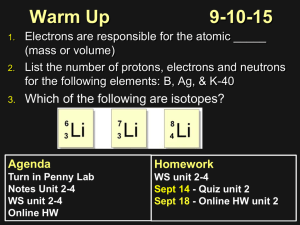
ARRANGEMENT OF ELECTRONS IN THE ATOM • Identify the first 20 elements in terms of the number of outer electrons. • Evaluate Bohr’s study of the spectra WHERE ARE THE ELECTRONS? Ernest Rutherford-electrons revolving in orbit. defied the law of physics-electrons spiralling around the nucleus would lose energy and spiral into the nucleus (hitting the atom). Niel’s Bohr-hypothesised a line spectra to show the electron arrangement. (white light broken up into a continuous spectrum) Light from a hydrogen discharge tube created narrow lines instead of a continuous spectra when passed through a prism. Hydrogen discharge tube is a glass tube filled with H at low pressure and an electric current is passed through. EMISSION LINE SPECTRUM Light is only emitted when an electric current is passed through hydrogen. When hydrogen was replaced by other elements, different spectra resulted. (fingerprint spectrum) An emission spectrometer is used to analyse steel for the presence of different elements. Examples of element colours in everyday life. Bohr’s atom 1. Electrons travel in orbits around the nucleus. 2) Electrons have a fixed amount of energy in any one orbit so the orbits are called energy levels. Each energy level has a definite value. The fixed amount of energy is called a quantum of energy. 3) Electrons don’t gain or lose energy if they are in one particular energy level. 4) When an atom absorbs energy, electrons jump from a lower energy level to a higher energy level. (higher the level the less stable)- further from nucleus 5) Energy is lost when electrons go from higher to lower levels but electrons can only fall to certain energy levels. (only a fixed amount of light energy is given off) N=1 N=2 What do you remember? KWL Today we will…. Define energy level, ground state and excited state. Homework P24 3.2 Bohr’s study of spectra To be continued. Ground state: where electrons in an atom occupy the lowest available energy levels where they have energies of fixed value. Emission spectra: Absorption spectra: Energy levels are represented by the letter N. The lowest energy level is n=1 Atoms normally exist in ground state i.e the electrons will have the lowest amount of energy possible and occupy the lowest energy orbitals. N=1 N=1 N=2 When a fixed amount of energy is absorbed, an electron will jump from n=1 to n=2 i.e a higher energy level. An energy level is defined as a fixed energy value that an electron in an atom may have. Ground state Electrons: Occupy lowest energy level. Have a fixed energy value. Need a specific amount of energy to jump from lower to higher energy levels to an excited state. The energy level an electron moves to depends on the atom. Excited state Energy absorbed=difference in energy between the lower level (ground state) and the higher level (excited state). Electrons in the excited state are unstable, the fall back down after a certain time period. The extra energy is released in the form of a photon of light with a definite amount of energy due to falling to a definite energy level. Light of a definite frequency is given off. Frequency of light: energy difference E2-E1=hf E2 higher energy level E1 lower energy level h= Planck’s constant f= Frequency of light emitted The energy difference s proportional to the frequency of light emitted. This frequency appears on an emission spectrum as a line of a particular colour. Each definite amount of energy gives rise to a line in the emission spectrum. Proves energy levels exist! Each element has a unique emission line spectrum because each element has a definite number of electrons and has its own arrangement of these electrons in energy levels. This is called electron configuration. True/False Was Bohr Correct? 1. After he realised the coloured line on the emission spectrum of hydrogen had its own frequency… 2. He Calculated mathematically the energy corresponding to n=1 n=2 etc. 3. Calculated the energy an electron would lose when falling from higher energy level to lower energy levels. 1. Used E2-E1=hf to calculate the frequency and wavelength of the coloured lines corresponding to the transitions. He calculated frequency from the above equation and velocity of light = frequency X wavelength allowed him to calculate wavelength Safety in the lab! Barium chloride in is harmful by ingestion and inhalation. Copper(II) chloride is toxic if swallowed, and is an eye and skin irritant. Lithium chloride n is harmful by ingestion, and is a severe eye and skin irritant. Potassium chloride is an eye irritant. Predict observe explain!! Copper sulfate: Blue-green Lithium chloride Lithium carbonate: Deep red Sodium chloride Sodium sulfate: Yellow Potassium chloride Strontium nitrate: Red Barium chloride Barium nitrate: Yellow-green Strontium chloride Potassium sulfate: Lilac Copper(II) chloride https://www.youtube.com/watch?v=Rf3 fKLLPpRY Atomic Absorption spectroscopy Scientists have discovered that atoms can absorb light. When white light is passed through a sample of a gaseous element, the light that comes out has certain wavelengths missing. i.e dark lines in the spectrum This is called an atomic absorption spectrum. The dark lines represent missing wavelengths and tell us what elements are present as the dark lines represent the electrons that have absorbed the light. The wavelengths that are absorbed correspond exactly to the wavelengths detected in the emission spectrum. What does that tell us? You need: White light Gaseous sample Prism AAS is an important tool used by scientists to determine certain elements and their concentrations.