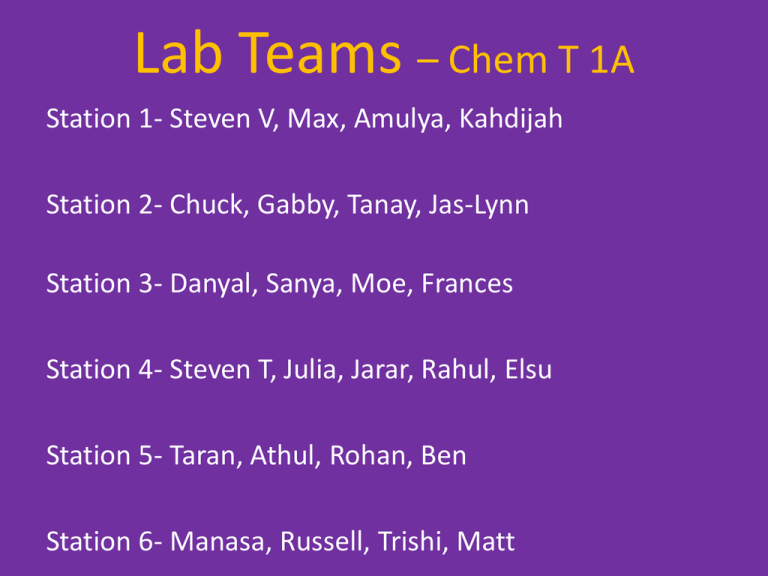
Lab Teams – Chem T 1A
Station 1- Steven V, Max, Amulya, Kahdijah
Station 2- Chuck, Gabby, Tanay, Jas-Lynn
Station 3- Danyal, Sanya, Moe, Frances
Station 4- Steven T, Julia, Jarar, Rahul, Elsu
Station 5- Taran, Athul, Rohan, Ben
Station 6- Manasa, Russell, Trishi, Matt
Lab Teams - Chem T 2A
Station 1- Manav, Hannah, Jaylend, Pooja
Station 2- Kenny, Fatumo, Ally, Jack
Station 3- Andrea, Abdul-Ahad, Amanda
Station 4- Kevin, Sam, Kailey
Station 5- Peter, Hussain, Anthony, Dhruv
Station 6- Janae, Jonathan, Samvit, Nidhi
Charges of Ions, Naming Ionic
Compounds
Atoms have NO overall charge
• Atoms are not charged, even though they are
made up of charged particles
• Atoms have an equal number of protons (positive
charge) and electrons (neg. charge) whose
charges exactly cancel.
• Ex: Helium (He)
– 2 protons, 2 neutrons, 2 electrons
+2
0
-2
= 0 total charge
Why do atoms bond??
• Atoms bond to be more stable!!
• Atoms join to form bonds so that each atom
has a full outermost energy level – by either
sharing or transferring electrons
• What is the number of electrons that atoms
want to have in their outermost shell?
Ionic Bonds
• Ionic bonds are formed
between oppositely
charged ions.
Ionic bonds are
formed by the
transfer of electrons
• Ionic bonds exist between
metal and non-metal and are
called Ionic Compounds
Covalent Bonds
• Covalent bonds are formed by the
sharing of electrons between atoms
Covalent Bonds exist between nonmetals and are called molecular
compounds
Atoms gain or lose electrons to
become IONS
Some Atoms Form Ions
•
An ion is an atom or group of atoms that has lost or
gained one or more electrons and has a net negative or
positive charge.
•
A lithium atom loses one electron to form a 1+ charged ion:
•
A fluorine atom gains one electron to form a -1 charged ion:
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Formation of Ions
• Cation: an atom loses one or more electrons and
becomes positively charged
Na – 1 electron Na+
+ +
• Anion: an atom gains one or more electrons and
becomes negatively charged
Cl + 1 electron Cl-
When will an element form a cation?
When will an element form an anion?
• An element wants to be stable. A stable electron arrangement
is one with eight electrons in the valence (or outer) shell.
Barium = 2 valence e- = Ba2+
cation
Iodine = 7 valence e- =
I- anion
What is the charge on the ions of the
following elements?
Hint: Use your Periodic Table!!
•
•
•
•
•
•
•
Sodium
Magnesium
Fluorine
Oxygen
Cesium
Phosphorus
Aluminum
OK Cool, Now that we
know that..
Writing and Naming Ionic
Formulas
Objectives
•Name simple ionic compounds.
•Write chemical formulas for simple ionic
compounds.
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Writing Ionic Formulas
What is the chemical formula for aluminum
fluoride?
1.List the elemental symbols for each ion (with the cation
first)
2. Write the charges as superscripts for each ion.
3. Criss-cross values of charges
4. Write the chemical formula, indicating with subscripts
how many of each ion are needed to make a neutral
compound.
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
What is the formula for the compound
made of Ca2+ cations and Cl1- anions?
Total charges of possible formulas are
shown:
CaCl:
Ca2Cl:
CaCl2:
1Ca2+ + 1 Cl1- (2+) + (1-) = 1+
2 Ca2+ + 1 Cl1- (2+) + (2+) + (1-) = 4+
1 Ca2+ + 2 Cl1- (2+) + (1-) + (1-) = 0
The only formula that is neutral is CaCl2
Use the crisscross method to find the
formula of an ionic compound
1.
Write the cation and anion symbols with ionic charges.
For calcium chloride,
Ca2+ Cl1-
2.
Make the number in the positive charge into a subscript
for the anion. Make the number in the negative charge
into a subscript for the cation. Ignore the – and + signs
Ca2+ Cl1Ca 1
3.
Cl 2
=
CaCl2
If necessary, reduce the resulting formula to its smallest
whole-number ratio. To do this, divide all subscripts by
the largest common factor
What is the formula for the
compound made of Mg2+ cations and
Br1- anions?
What is the formula for the
compound made of Al3+ cations and
S2- anions?
Practice
• Write ionic formulas for the following ionic
compounds:
• Lithium oxide
• Beryllium chloride
• Magnesium oxide
• Sodium Chloride
• Lithium Nitrate
• Aluminum Sulfide
Naming Binary Ionic Compounds
• There are two types of binary ionic compounds:
– Type I: Metal only forms one cation (not a transition metal)
– Type 2: Metal can form two (or more) cations that have different
charges (transition metal)
• Naming Type I Binary Compounds
– Name the cation first and the anion second
– Replace the ending of the anion with –ide to it.
• Examples:
– NaCl
• Sodium Chloride
– CaO
• Calcium Oxide
– ZnCl2
• Zinc Chloride
Important!!
• Some metal atoms form ions with different
charges under different conditions. To specify
the charge for these ions, Roman numerals are
used in parentheses after the name of the
metal. These include: copper, lead, iron,
mercury.
• Check your ion reference sheet!!
Naming Binary Ionic Compounds with
Cations of Varying Charge
– Name the cation first
– Write charge of cation as roman numeral in
parentheses
– Anion with the –ide ending
• Fe2O3
– Iron (III) Oxide
• AuCl
– Gold (1) Chloride
• CuBr2
• Copper (II) Bromide
Naming Binary Ionic Compounds –
Ions of varying charge
Some cation names must show their charge.
• Iron can form two different cations. Fe2O3 is made of
Fe3+ ions, so it is named iron(III)oxide. FeO is made of
Fe2+ ions, so it is named iron(II) oxide.
To determine the charge of a transition metal cation,
look at the total charge of the compound.
• You can tell that the iron ion in Fe2O3 has a charge of 3+
because the total charge of the compound must be
zero, and an oxide ion, O2–, has a a charge of 2–.
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Try these!
•
•
•
•
CuCl
CuCl2
PbF2
PbF4
Write the Formula for Binary Ionic Compounds
with Transition Metals
Iron (111) Sulfide
Tin (11) Chloride
Copper(1)Oxide
Tin (1V) Oxide
Tin(11) Oxide
Naming Tertiary Compounds
• Naming Tertiary Ionic Compounds
– Name cation
– Indicate the charge if cation has varying charges
– Name the polyatomic ion
A polyatomic ion is a group of two or more atoms covalently
bonded or metallically bonded together to function as a
single ion (has a charge associated with it).
An example being the hydroxide ion, OH-. The oxygen and
hydrogen are covalently bonded together, and function as
a single anion with a 1- charge.
Polyatomic Ions
BrO3- bromate
IO3- iodate
CO2-2 carbonite
Naming Tertiary Ionic Compounds
• Check if the cation requires that you show charge
• Name cation
• Name anion (polyatomic)
• Examples
– Na2SO4
– Fe(NO3)3
– (NH4 )OH
– Cu(NO2)2
Writing Formulas for Tertiary Ionic
Compounds
Potassium Hydroxide
Magnesium Sulfate
Iron (111) Nitrate
Zinc (11) Phosphate
CaBr2
SnO
Pb(CO3)2
WRITE THE CHEMICAL FORMULA
Don’t use parentheses unless necessary
Mercury (II) sulfide
Cobalt (III) nitrate
Magnesium phosphate
Tin (II) Nitride
Copper (I) Sulfide




