Slide 1
advertisement

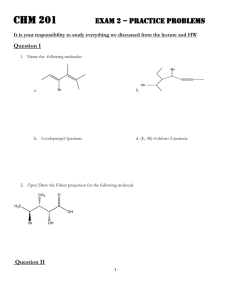
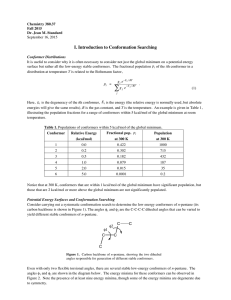
Chemistry 2500 Conformations of Organic Molecules – Fall 2009 Conformations of Organic Molecules A conformation is an instantaneous orientation of atoms. Different conformations arise from bond rotation, NOT from bond cleavage. H H H C H = C H H H H HH H H C H H H = C H Sawhorse H H H H H H H H Newman Projection Conformers – Ethane eclipse d staggere d HH H H H H H HH H H H H H H H H H E H H H H H 60 H 120 180 Dihedral or Torsional Angle Which conformer is favoured? 240 Conformations - Butane Build a model of butane. Looking down the C2-C3 bond, draw the Newman Projections of the four different conformers of butane. What are the relative energies of these conformers? Conformers - Cyclohexane Cyclohexane is the only ring under 14 carbon atoms that is relatively free from strain. The two most common conformations of cyclohexane are the chair conformation and the boat conformation. H H H C H H H H C C C C H H H C H H C C H chair C C H H H H C C H H H H boat H H Conformers - Cyclohexane What are the bond angles around each carbon atom in the chair conformation? Look down any carbon-carbon bond. What are the relative positions of adjacent hydrogen atoms? Notice that the hydrogen atoms are found in one of two positions: H H H H C H H C H H C C C H C H H H H H H H H H H H Conformers - Cyclohexane There are two different chair conformations that interconvert through a process called a ring flip. H H H H H C H H H H C H H H H H C H H H C H H H What are the consequences of a ring flip? H H H H Conformers – Substituted Cyclohexane What happens when we substitute one of the hydrogen atoms for another group, say methyl? H CH 3 H H H H H H H H H H CH 3 H H H H H H H H H H CH 3 H H H H H H H H H H CH 3 H H H Are these two conformations equivalent? H H Conformers – Substituted Cyclohexane H H H H X H H H HH H H H H H H [Xeq] K= H X H H H Xax. H [Xax] ΔG° = -RTlnK H H Xeq. X K ΔG° (KJ/mol) -H 1 0 -CH3 19 -7.3 -CH2CH3 21 -7.5 -C(CH3)3 3300 -20 -F 1.7 -1.3 -Cl 3 -2.7 -Br 3 -2.7 -I 2.3 -2.1 Conformers – Substituted Cyclohexane Rationalize the relative ΔG° values for the following pairs of groups: ◦ -CH3 vs -C(CH3)3 ◦ -CH3 vs -Cl ◦ -Cl vs -F ◦ -Cl vs -I Conformers – Disubstituted Cyclohexane Consider the two chair conformers for cis-1,2-dimethylcyclohexane and trans-1,2-dimethylcyclohexane CH 3 CH 3 CH 3 CH 3 H H H H CH 3 H H CH 3 CH 3 H Is one conformer favoured over the other? CH 3 H Conformers – Disubstituted Cyclohexane Now consider the two chair conformers for cis-1,3dimethylcyclohexane and trans-1,3-dimethylcyclohexane. CH 3 CH3 H3 C CH 3 H H H H CH 3 H H CH 3 H CH 3 CH3 Is one conformer favoured over the other? H Conformers – Disubstituted Cyclohexane What is the most stable chair conformer for cis-1-tbutyl-4methylcyclohexane. CH 3 CH3 H3 C C CH 3 H 3C CH 3 H C H CH 3 H 3C H H What is the most stable conformer for menthol? OH Conformers – Cycloalkanes and Chirallity Remember, ring flips are like bond rotations – they only change the conformation of the molecule. Therefore, you are allowed to do ring flips to determine if your molecule is chiral or not. Convince yourself that cis-1,2-dichlorocyclohexane is achiral. Convince yourself that trans-1,2-dichlorocyclohexane is chiral. Conformers – Smaller Cycloalkanes Six-membered rings are prevalent in nature because they are relatively free from: ◦ torsional strain: eclipsed bonds and ◦ angle strain: bonds angles that deviate from those predicted by VSEPR theory Build cyclopentane, cyclobutane, and cyclopropane. Is there torsional strain? Angle strain? What do you think would be the most stable conformer for each of these rings? Use the amount of strain to rank these rings from most to least stable.