Beryllium Chloride: Properties & Structure (BeCl2)
Beryllium chloride is an inorganic compound with the formula BeCl
2
. It is a colourless, hygroscopic solid that dissolves well in many polar solvents.
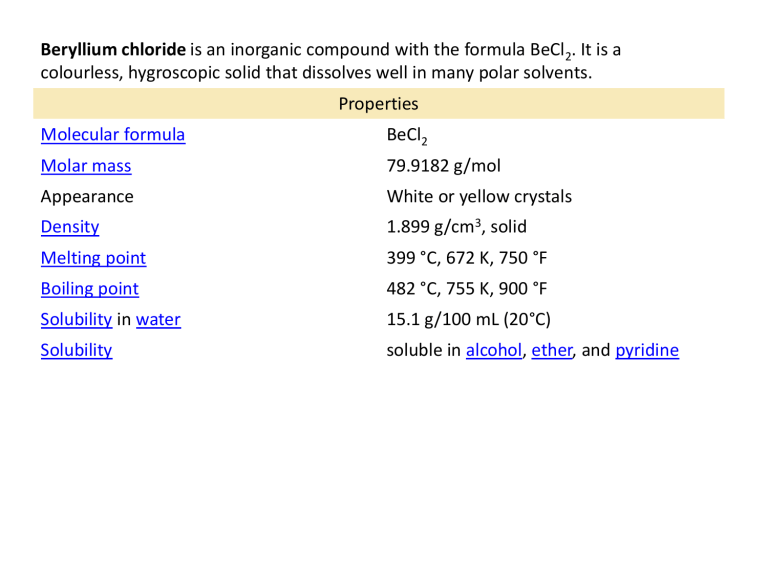
Molecular formula
Molar mass
Appearance
Density
Melting point
Boiling point
Solubility
Solubility in water
Properties
BeCl
2
79.9182 g/mol
White or yellow crystals
1.899 g/cm 3 , solid
399 °C, 672 K, 750 °F
482 °C, 755 K, 900 °F
15.1 g/100 mL (20°C) soluble in alcohol , ether , and pyridine
Beryllium chloride is covalent
The facts
Physical properties
Beryllium chloride, BeCl
2
, melts at 405 °C and boils at 520°C.
That compares with 714 °C and 1412°C for magnesium chloride.
Notice how much dramatically lower the boiling point of beryllium chloride is compared with magnesium chloride. The much higher boiling point of magnesium chloride is what you might expect from the strong forces between the positive and negative ions present.
Because its boiling point is much lower, it follows that beryllium chloride can't contain ions - it must be covalent. On the other hand, the melting point is quite high for such a small covalent molecule.
The structure of beryllium chloride
As a gas . . .
Beryllium chloride, BeCl
2
, is a linear molecule with all three atoms in a straight line. Showing only the outer electrons:
Beryllium chloride is known as an electron-deficient compound because it has the two empty orbitals at the bonding level.
As a solid . . .
If it had this same simple structure as a solid, you would expect the melting point to be much lower than it actually is. It is a very small molecule, and so the intermolecular attractions would be expected to be fairly weak
In the solid, the BeCl
2 molecules polymerise to make long chains.
They do this by forming coordinate bonds (dative covalent bonds) between lone pairs on chlorine atoms and adjacent beryllium atoms.
The diagram shows a simple dimer - the start of the polymerisation process.
You can see from the last diagram that the beryllium atoms are still electron deficient. The process continues. The next diagram shows the coordinate bonds in the conventional way using arrows. The arrow goes from the atom which is supplying the pair of electrons to the atom with the empty orbital.
The solid is a 1-dimensional polymer consisting of edgeshared tetrahedra. In the gas phase, it exists both as a linear monomer ad a bridged dimer with two bridging chlorine atoms where the beryllium atom is 3-coordinate. The linear shape of the monomeric form is as predicted by VSEPR theory.



