Complete the ladder Conversion Practice
advertisement
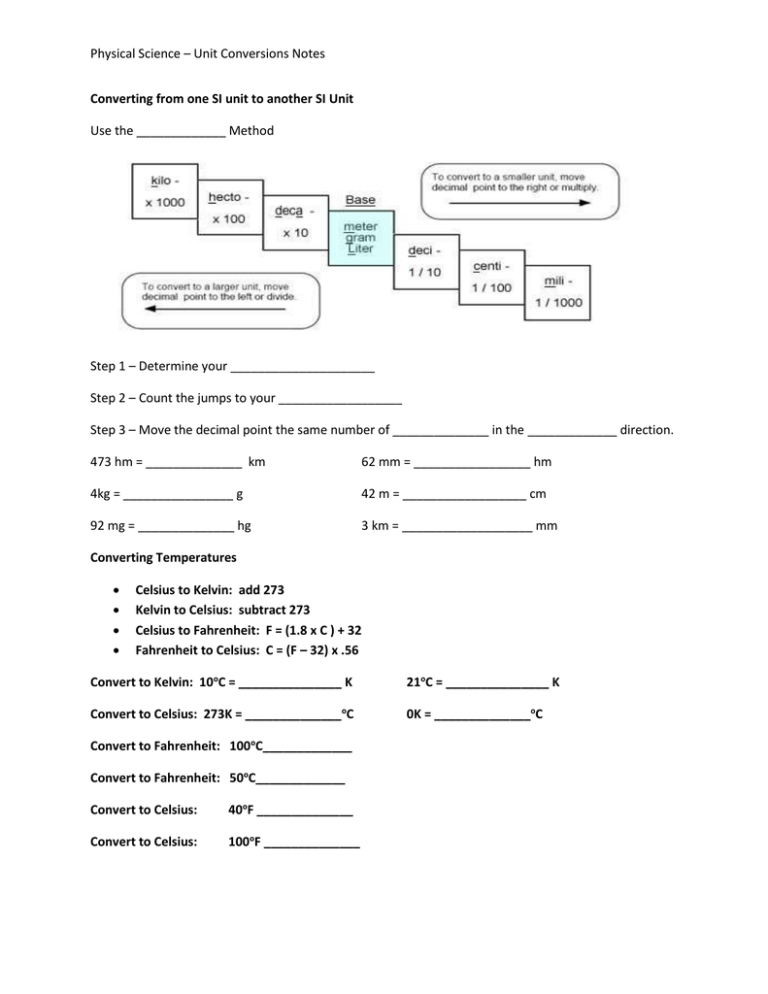
Physical Science – Unit Conversions Notes Converting from one SI unit to another SI Unit Use the _____________ Method Step 1 – Determine your _____________________ Step 2 – Count the jumps to your __________________ Step 3 – Move the decimal point the same number of ______________ in the _____________ direction. 473 hm = ______________ km 62 mm = _________________ hm 4kg = ________________ g 42 m = __________________ cm 92 mg = ______________ hg 3 km = ___________________ mm Converting Temperatures Celsius to Kelvin: add 273 Kelvin to Celsius: subtract 273 Celsius to Fahrenheit: F = (1.8 x C ) + 32 Fahrenheit to Celsius: C = (F – 32) x .56 Convert to Kelvin: 10oC = _______________ K 21oC = _______________ K Convert to Celsius: 273K = ______________oC 0K = ______________oC Convert to Fahrenheit: 100oC_____________ Convert to Fahrenheit: 50oC_____________ Convert to Celsius: 40oF ______________ Convert to Celsius: 100oF ______________ Physical Science – Unit Conversions Notes Complete the ladder Conversion Practice Name_________________________ Try these conversions, using the ladder method. 1000 mg = _______ g 1 L = _______ mL 160 cm = _______ mm 14 km = _______ m 109 g = _______ kg 250 m = _______ k 1000 mL = _______ L 1 g = _______ mg 160 cL = _______ mL 14 kg = _______ g 1090 L = _______ kL 50 mg = _______ kg Dimensional Analysis (also known as the “unit factor” or the “factor label” method) Given a conversion factor, such as 1 inch = 2.54 centimeters, I can rewrite this two ways: 1 2 I also know that I can multiply any value by _________ and not change its value. I can use this fact to convert from one unit to another. Convert 5 in to cm__________________ Convert 10 in to cm__________________ Convert 12 cm to in___________________ Convert 100 cm to in__________________


![Temperature Notes [9/22/2015]](http://s3.studylib.net/store/data/006907012_1-3fc2d93efdacd086a05519765259a482-300x300.png)

