The entropy (ΔS) - s3.amazonaws.com
advertisement

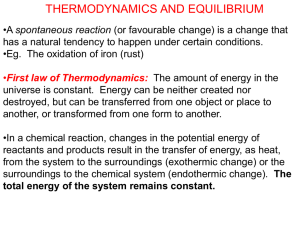
Bond Enthalpies How does a chemical reaction have energy? Bond Energy Energy required to make/break a chemical bond Endothermic reactions Products have more energy than reactants More energy to BREAK bonds Exothermic reactions Reactants have more energy than products More energy to FORM bonds Bond Enthalpy Focuses on the energy/heat between products and reactants as it relates to chemical bonding Amount of energy absorbed to break a chemical bond--amount of energy released to form a bond. Multiple chemical bonds take more energy to break and release more energy at formation Amount of energy absorbed = amount of energy released to break chemical bond to form a chemical bond Calculating ΔHrxn. by bond th enthalpies (4 method) Least accurate method ΔH = ΣBE (bonds broken) - ΣBE (bonds formed) Example 1: Using average bond enthalpy data, calcaulate ΔH for the following reaction. CH4 + 2O2 CO2 + 2H2O ΔH = ? Bond Average Bond Enthalpy C-H 413 kJ/mol O=O 495 kJ/mol C-O 358 kJ/mol C=O 799 kJ/mol O-H 467 kJ/mol Entropy Spontaneous vs. Nonspontaneous 1) Spontaneous Process Occurs WITHOUT help outside of the system, natural Many are exothermic—favors energy release to create an energy reduction after a chemical reaction Ex. Rusting iron with O2 and H2O, cold coffee in a mug Some are endothermic Ex. Evaporation of water/boiling, NaCl dissolving in water Spontaneous vs. Nonspontaneous 2) Nonspontaneous Process REQUIRES help outside system to perform chemical reaction, gets aid from environment Ex. Water cannot freeze at standard conditions (25°C, 1atm), cannot boil at 25°C **Chemical processes that are spontaneous have a nonspontaneous process in reverse ** Entropy (S) Measure of a system’s disorder Disorder is more favorable than order ΔS = S(products) - S(reactants) ΔS is (+) with increased disorder State function Only dependent on initial and final states of a reaction Ex. Evaporation, dissolving, dirty house Thermodynamic Laws 1st Law of Thermodynamics Energy cannot be created or destroyed 2nd Law of Thermodynamics The entropy of the universe is always increasing. Naturally favors a disordered state When does a system become MORE disordered from a chemical reaction? (ΔS > 0) 1) Melting 2) Vaporization 3) More particles present in the products than the reactants 4C3H5N3O9 (l) 6N2 (g) + 12CO2 (g) + 10H2O (g) + O2 (g) 4) Solution formation with liquids and solids 5) Addition of heat When does a system become LESS disordered from a chemical reaction? (ΔS < 0) 1) Solution formation with liquids and gases 3rd Law of Thermodynamics The entropy (ΔS) of a perfect crystal is 0 at a temperature of absolute zero (0°K). No particle motion at all in crystal structure All motion stops How do we determine if a chemical reaction is spontaneous? 1) Change in entropy (ΔS) 2) Gibbs Free Energy (ΔG) Change in entropy (ΔS) For a chemical reaction to be spontaneous (ΔST > 0), there MUST be an increase in system’s entropy (Δssys> 0) and the reaction MUST be exothermic (Δssurr > 0). Exothermic reactions are favored, NOT endothermic reactions. Exothermic (ΔH < 0, ΔS > 0) Endothermic (ΔH > 0, ΔS < 0) ΔST = Δssys + Δssurr If ΔST > 0, then the chemical reaction is spontaneous Example 1: Will entropy increase or decrease for the following? a) N2 (g) + 3H2 (g) 2NH3 (g) b) 2KClO3 (s) 2KCl (s) + 3O2 (g) c) CO(g) + H2O(g) CO2 (g) + H2 (g) d) C12H22O11 (s) C12H22O11 How do we calculate the entropy change (ΔS) in a chemical reaction? Same method as using the enthalpies of formation to calculate ΔH and use the same table. aA + bB cC + dD ΔS° =[c (ΔS°C) + d(ΔS°D)] - [a (ΔS°A) + b (ΔS°B)] Example 2: Calculate ΔS° for the following reaction at 25°C…. 4HCl(g) + O2 (g) 2Cl2 (g) + 2H2O (g) Homework Finish problems #16-19 on enthalpy worksheet. pp. 742-473 #19, 23, 26, 27