Chapter16
advertisement
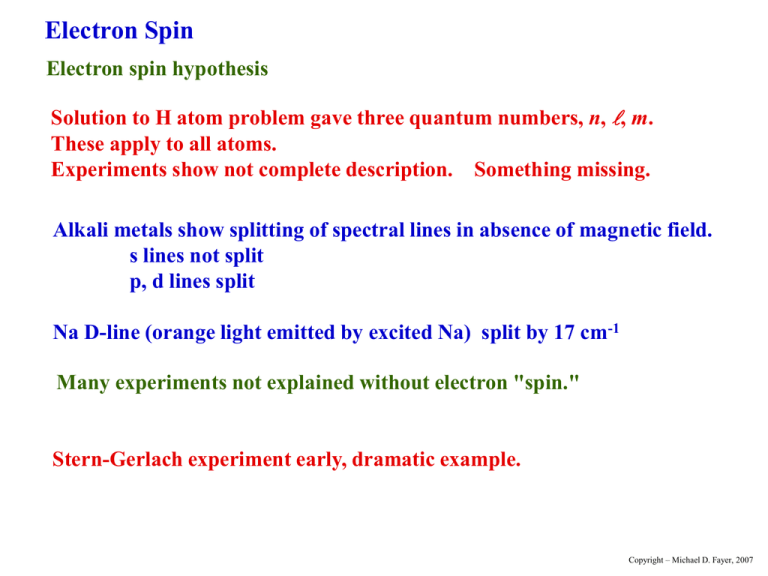
Electron Spin Electron spin hypothesis Solution to H atom problem gave three quantum numbers, n, , m. These apply to all atoms. Experiments show not complete description. Something missing. Alkali metals show splitting of spectral lines in absence of magnetic field. s lines not split p, d lines split Na D-line (orange light emitted by excited Na) split by 17 cm-1 Many experiments not explained without electron "spin." Stern-Gerlach experiment early, dramatic example. Copyright – Michael D. Fayer, 2007 Stern-Gerlach experiment glass plate Magnet ms oven baffles Ag Ag beam N +1/2 S -1/2 Beam of silver atoms deflected by inhomogeneous magnetic field. Single unpaired electron - should not give to lines on glass plate. s-orbital. No orbital angular momentum. No magnetic moment. Observed deflection corresponds to one Bohr magneton. Too big for proton. Copyright – Michael D. Fayer, 2007 Assume: Electron has intrinsic angular momentum called "spin." 1 s 2 11 2 S s ms 1 2 2 square of angular momentum operator projection of angular momentum on z-axis S z s ms 1 2 s s ms s ms s e /2mc s 2 e mc e 2mc Electron has magnetic moment. One Bohr magneton. Charged particle with angular mom. Ratio twice the ratio for orbital angular momentum. Copyright – Michael D. Fayer, 2007 Electronic wavefunction with spin ( x, y, z ) ( x , y , z , ms ) ms with spin 1 2 spin angular momentum kets s ms ( ms ) 11 22 1 1 2 2 11 1 1 , 22 2 2 Copyright – Michael D. Fayer, 2007 Electronic States in a Central Field Electron in state of orbital angular momentum Y m ( , ) m ms label as orbital angular momentum quantum number spin angular momentum quantum number (1/2), (1/2) m ms Y m ms product space of angular momentum (orbital and spin) eigenvectors Copyright – Michael D. Fayer, 2007 m ms Simultaneous eigenvectors of L L 2 2 Lz S 2 Sz 1 Lz m constant - eigenvalues of S2 are s(s + 1), s = 1/2 S 2 3 4 S z ms 2 2 1 linearly independent functions of form m ms . Copyright – Michael D. Fayer, 2007 Example - p states of an electron Orbital functions =1 ( r , , ) R( r )Y m ( , ) radial spherical harmonics angular momentum part Y11 11 Y10 10 j1m1 j1m1 Y11 1 1 j1m1 spin functions j1m1 m 11 22 j2m 1 1 2 2 j2m 2 2 j2 m2 s ms Copyright – Michael D. Fayer, 2007 In the m1m2 representation s m ms m ms 1 1 1 Y11 1 1 1 2 2 2 Y11 1 1 1 1 2 2 1 Y10 1 1 2 0 0 Y10 1 1 2 0 Y11 1 1 1 1 2 2 Y11 1 1 1 1 1 1 2 2 2 1 2 1 2 1 2 0 1 1 2 1 2 1 2 Each of these is multiplied by R(r). j1 j2 s Copyright – Michael D. Fayer, 2007 Total angular momentum - jm representation Two states of total angular momentum j j1 j2 s 3 2 j j1 j2 1 j1 j2 s 1 2 The jm kets are 33 22 31 22 3 1 2 2 3 3 2 2 11 22 1 1 2 2 Copyright – Michael D. Fayer, 2007 The jm kets can be obtained from the m1m2 kets using the table of Clebsch-Gordon coefficients. 11 2 1 1 1 1 0 For example 22 3 2 3 2 jm Table of Clebsch-Gordon Coefficients 3 2 3 2 1 2 3 2 1 2 1 2 1 2 1 3 2 3 0 1 2 2 3 0 2 1 2 3 1 3 1 1 2 1 3 2 j1 = 1 j 2 = 1/2 1 2 1 1 1 1 2 m1 m2 m1 m2 3 2 1 2 1 2 1 2 3 2 j 3 2 m 1 1 3 3 1 m1 m2 Copyright – Michael D. Fayer, 2007 Spin-orbit Coupling An electron, with magnetic moment moves in the electric field of rest of atom (or molecule). W E V classical energy of magnetic dipole moving in electric field E with velocity V energy eS spin magnetic moment of electron 2mc Then W E V S 2mc e Using e E grad Coulomb potential (usually called V, but V is velocity) and p mV Copyright – Michael D. Fayer, 2007 1 W ( grad p) S 2 2m c Hydrogen like atoms ze 2 Coulomb potential 4 0 r unit vector ze 2 r ze 2 grad r vector derivative of potential 2 3 4 0 r r 4 0 r Substituting ze 2 1 H so r p S 3 2 8 0 m c r (r p) L H so operates on radial part of wavefunction orbital angular momentum ze 2 1 L S 3 2 8 0 m c r operates on orbital ang. mom. part of wavefunction operates on spin ang. mom. part of wavefunction Copyright – Michael D. Fayer, 2007 One unpaired electron – central field (Sodium outer electron) V (r ) V (r ) 1 grad V ( r ) r r r H so result of operating on radial part of wavefunction 1 1 V (r ) L S a(r ) L S 2 2m c r r Many electron system H so 1 V ( ri ) 1 Li S i ai ( r ) Li S i 2 2m c i ri ri i Ignore terms involving, Li S j , the orbital angular momentum of one electron with the spin of another electron. Extremely small. Copyright – Michael D. Fayer, 2007 Spin-orbit coupling piece of Hamiltonian H so a ( r ) L S H-like atom – spatial part of operator ze 2 1 H so ( r ) 8 0 m 2 c r 3 E (r ) (r ) H so (r )d Normalization constant (eq.7.63) contains z 3 /2 E ( r ) z z 3 /2 z 3 /2 H – little S.O. coupling Br – sizable S.O. coupling a( r ) z 4 heavy atom effect similar for all atoms and molecules Heavy atom effect and external heavy atom effect important in many processes. Copyright – Michael D. Fayer, 2007 H so a ( r ) L S The a(r) part is independent of the angular momentum states. Consider L S L S L x S x L y S y Lz S z Rearranging the expressions for the angular momentum raising and lowering operators 1 S x S S 2 1 S y S S 2i 1 L x L L 2 1 L y L L 2i Substituting L S Lz S z 1 L S L S 2 Copyright – Michael D. Fayer, 2007 Spin-Orbit Coupling in the m1m2 representation L S Lz S z 1 L S L S 2 not diagonal in the m1m2 representation States in the m1m2 representation 1 1 2 1 1 2 0 1 2 0 1 2 1 1 2 1 1 2 The spin-orbit coupling Hamiltonian matrix in the m1m2 representation is a 6 6. Calculating the matrix elements Kets are diagonal for the Lz S z piece of the Hamiltonian. For example 1 1 1 1 2 2 2 m ms m ms Lz S z 1 Lz brings out m and Sz brings out ms . Copyright – Michael D. Fayer, 2007 1 L S L S 2 Apply not diagonal 1 L S L S to the six kets. 2 Examples 1 1 L S L S 1 0 2 2 can’t raise above largest values 1 1 2 1 L S L S 1 0 2 2 2 2 Left multiply by 0 0 1 2 1 1 1 2 L S L S 1 2 2 2 2 matrix element Copyright – Michael D. Fayer, 2007 1 H SO a( r ) L z S z L S L S 2 11 2 Hso= 1 12 1 1 2 1 0 2 a(r) 1 0 2 1 1 2 1 1 2 1 1 2 1 2 01 2 put kets along top 0 1 2 1 1 2 1 1 2 1 2 put bras down left side 2 2 2 2 0 0 2 2 2 2 1 2 1 2 example 0 1 1 1 2 L S L S 1 2 2 2 2 Matrix is block diagonal. Copyright – Michael D. Fayer, 2007 Matrix – Block Diagonal Each block is independent. Diagonalize separately. Form determinant from block with (eigenvalue) subtracted from diagonal elements. 1 2 2 2 2 2 0 The two blocks are the same. Only need to solve one of them. The two 11 blocks are diagonal – eigenvalues. Expand the determinant. Solve for . Each block multiplied by a(r). 1/2 2 1/2 0 1/4 3 / 4 [1/ 2 or 1] 1 2 a(r ) 1 a(r ) The two 22 blocks each give these results. The two 11 blocks each give 1/2a(r). Therefore, 4 eigenvalues of 1/2a(r). 2 eigenvalues of -1a(r). Copyright – Michael D. Fayer, 2007 Energy level diagram four-fold degenerate 0.5a(r) fluorescence no spin-orbit coupling 3p E = 16,980 cm-1 0 E two-fold degenerate -1a(r) Na D-line (3p to 3s transition) split by 17 cm-1. 17 cm-1 = 3/2 a(r). a(r) = 11.3 cm-1. Ratio a(r ) 7 104 E 3s Ratio small, but need spin-orbit coupling to explain line splitting. Copyright – Michael D. Fayer, 2007 Spin-orbit Coupling in the jm representation The jm kets are 33 22 31 22 3 1 2 2 3 3 2 2 11 22 1 1 2 2 However a( r ) L S is in the m1m2 representation. Can’t operate HSO directly on the jm kets. Use table of Clebsch-Gordan coefficient to take jm kets into m1m2 rep., operate, then convert back to jm rep. Copyright – Michael D. Fayer, 2007 Table of Clebsch-Gordan Coefficients 3 2 3 2 1 2 3 2 1 2 1 2 1 2 1 3 2 3 0 1 2 2 3 0 2 1 2 3 1 1 2 1 3 j1 = 1 j 2 = 1/2 1 2 1 1 3 2 1 2 1 2 1 2 3 2 j 3 2 m 1 1 3 1 3 2 3 1 1 2 1 m1 m2 jm Example 11 22 m1 m2 m1 m2 2 1 1 1 1 0 3 2 3 2 Copyright – Michael D. Fayer, 2007 Operate a( r ) L S a(r ) L S on 3 3 22 L S Lz S z 1 L S L S 2 33 1 1 1 a(r ) L S 1 a(r ) 1 2 2 2 2 2 Found already that this is diagonal. Can’t raise above largest values. Therefore, a(r ) L S 33 22 33 1 33 a(r ) 2 2 2 2 2 is an eigenket of a( r ) L S with eigenvalue 1/2a(r) 33 1 1 2 2 2 1 1 2 forms 11 block in matrix. Eigenvector Copyright – Michael D. Fayer, 2007 Consider a( r ) L S operating on 2 11 1 1 1 1 0 22 2 3 2 3 jm m 1 m2 m1 m2 11 22 from Clebsch-Gordan table 1 1 1 11 1 2 1 0 a( r ) L S a ( r ) L z S z L S L S 2 3 2 22 2 3 1 1 1 2 1 1 2 1 1 2 0 0 1 a( r ) 1 0 a(r ) 0 2 2 3 2 3 2 2 3 2 2 11 1 1 1 1 a ( r ) 1 0 1 a ( r ) 22 2 3 2 3 a( r ) L S 11 11 1 a ( r ) 22 22 eigenvector with eigenvalue –1a(r) Copyright – Michael D. Fayer, 2007 Applying HSO to all jm kets Eigenkets The jm representation kets are the eigenkets because HSO couples the orbital and spin angular momenta. Coupled representation eigenkets. The jm kets 33 22 31 22 11 22 1 1 2 2 3 1 2 2 -1.0a(r) eigenvalues - +0.5a(r) eigenvalues - -1.0a(r) +0.5a(r) Na - 3p unpaired electron 3 3 2 2 33 22 31 22 11 22 1 1 2 2 3 1 2 2 3 3 2 2 0 Copyright – Michael D. Fayer, 2007 The jm representation kets will be the eigenkets whenever a term in Hamiltonian couples two types of angular momenta. Examples: Hyperfine Interaction - I S Coupling of electron spin and nuclear spin. Small splitting in Na optical spectrum. Structure in ESR spectra. NMR - nuclear spins coupled to each other. Electronic triplet states - coupling of two unpaired electrons. Copyright – Michael D. Fayer, 2007 Have diagonalized HSO in m1m2 representation. Have shown that jm kets are eigenkets. A unitary transformation U m m2 will take the non-diagonal matrix H so1 in the m1m2 representation into the diagonal matrix H sojm in the jm representation. H so U H so1 2 U jm mm 1 U is the matrix of Clebsch-Gordan Coefficients. Copyright – Michael D. Fayer, 2007 11 2 1 1 2 Both are block diagonal. Only need to work with corresponding blocks. 1 2 1 2 01 2 0 1 2 1 0 2 1 1 2 1 1 2 0 1 1 1 2 1 2 2 2 2 2 m1m2 matrix 0 0 2 2 2 2 1 2 1 2 3 2 3 2 1 2 3 2 1 2 1 2 1 2 1 3 2 3 0 1 2 2 3 1 0 21 2 3 1 1 2 1 3 j1 = 1 j 2 = 1/2 1 2 1 1 1 2 1 2 1 12 Hso= a(r) 1 1 21 3 2 1 2 1 2 1 2 3 2 j 3 2 m 1 Table of Clebsch-Gordan coefficients 3 1 3 2 3 1 m1 m2 Copyright – Michael D. Fayer, 2007 Multiplying the 2×2 blocks U 1 3 1 3 2 3 2 3 H SO 2 1 3 2 1 2 3 2 2 1 1 3 2 3 1 1 2 3 2 3 1/ 2 0 0 1 2 2 0 U 1 3 2 3 1 2 3 1 3 2 3 1 3 diagonal matrix with eigenvalues on the diagonal Copyright – Michael D. Fayer, 2007 Electron Spin, Antisymmetrization of Wavefunctions, and the Pauli Principle In treating the He atom, electron spin was not included. Electron - particle with intrinsic angular momentum, spin S 1/ 2 m s 1/ 2 1/2 1/2 1/2 1/2 Copyright – Michael D. Fayer, 2007 Excited States of He neglecting Spin Ground State of He - perturbation theory H' e2 perturbation 4 0 r12 Zeroth order wavefunctions (any level) Product of H atom orbitals n 1 1 m1 (1) n2 2 m2 (2) n1 1m1 (1) n2 2 m2 (2) (1) electron 1; coordinates ( r1 , 1 , 1 ) (2) electron 2; coordinates ( r2 , 2 , 2 ) Copyright – Michael D. Fayer, 2007 Zeroth order energy 1 1 En01n2 4 Rhc 2 2 n1 n2 e4 R 2 3 8 0 h c - reduced mass of H atom First excited state energy zeroth order E 0 5 Rhc since n1 1 and n2 2 n2 1 and n1 2 same energy Copyright – Michael D. Fayer, 2007 First Excited Configuration States corresponding to zeroth order energy - 8 fold degenerate 1 s 1 2 s 2 1 s 1 2 p y 2 2 s 1 1s 2 2 p y 1 1 s 2 1 s 1 2 p x 2 1 s 1 2 pz 2 2 p x 1 1 s 2 2 pz 1 1 s 2 All have same zeroth order energy. All have n1 1 and n2 2 or n2 1 and n1 2 Copyright – Michael D. Fayer, 2007 Degenerate perturbation theory problem System of equations (matrix) Form determinant 1 1 1s(1)2s(2) 2 2s(1)1s(2) 3 1s(1)2p x (2) 4 2 p x (1)1s(2) 2 3 4 5 6 7 8 Js E' Ks Ks Js E' Jpx E' Kpx Kpx Jpx E' 5 1s(1)2 p y (2) 6 2p y (1)1s(2) =0 Jpy E ' Kpy Kpy Jpy E' 7 1s(1)2p z (2) Jpz E' 8 2 p z (1)1s(2) Kpz Kpz Jpz E' E' - eigenvalues J's - diagonal matrix elements K's - off diagonal matrix elements Copyright – Michael D. Fayer, 2007 J s 1s(1)2 s(2) e2 4 o r12 K s 1s(1)2 s(2) e2 4 o r12 J px 1s(1)2 p x (2) py 2 s(1)1s(2) d 1d 2 e2 4 o r12 K px 1s(1)2 p x (2) J 1s(1)2 s(2) d 1d 2 e2 4 o r12 1s(1)2 p x (2) d 1d 2 2 p x (1)1s(2) d 1d 2 , K p y , J p z , K p z replace x with y and z Copyright – Michael D. Fayer, 2007 J's - Coulomb Integrals Classically represent average Coulomb interaction energy of two electrons with probability distribution functions 1s(1) 2 for 2 s(2) and 2 Js K's - Exchange Integrals No classical counter part. Product basis set not correct zeroth order set of functions. Exchange - Integrals differ by an Exchange of electrons. K s 1s(1)2 s( 2) e2 4 o r12 2 s(1)1s( 2) d 1d 2 Copyright – Michael D. Fayer, 2007 Other matrix elements are zero. Example e2 1s(1)2s(2) 4 r 1s(1)2 pz (2) d 1d 2 0 o 12 odd function - changes sign on inversion through origin Other functions are even. Integral of even function times odd function over all space = 0. Copyright – Michael D. Fayer, 2007 Eigenvalues (E') E J s K s 1) Set determinant = 0 2) Expand Four 2×2 blocks 3 blocks for p orbitals identical Js Ks J pi K pi triple root, px, py, pz J pi K pi triple root, px, py, pz Energy level diagram 1P triply degenerate 1S2P first excited states n1,n2 = 1,2 3P 1S2S triply degenerate 1S 3S no off-diagonal elements 1S2 ground state n1,n2 = 1,1 1S S configuration more stable. S orbital puts more electron density close to nucleus greater Coulombic attraction. Copyright – Michael D. Fayer, 2007 Eigenfunctions E J s K s 1 1s(1)2s(2) 2 s(1)1s(2) 2 E J s K s 1 1s(1)2s(2) 2 s(1)1s(2) 2 E J pi K pi E J pi K pi 1 1s(1)2 pi (2) 2 pi (1)1s(2) 2 i x, y, z 1 1s(1)2 pi (2) 2 pi (1)1s(2) 2 Copyright – Michael D. Fayer, 2007 Symmetric and Antisymmetric Combinations + combination symmetric. Symmetric with respect to the interchange of electron coordinates (labels). P 1 P is the permutation operator. P interchanges the labels of two electrons (labels - coordinates) Applying P to the wavefunction gives back identical function times 1. Copyright – Michael D. Fayer, 2007 - combination antisymmetric. Antisymmetric with respect to the interchange of electron coordinates (labels). Switching coordinates of two electrons gives negative of function. P P 1 1s(1)2 s(2) 2 s(1)1s(2) 2 1 1s(2)2 s(1) 2 s(2)1s(1) 2 1 1 1s(1)2 s(2) 2 s(1)1s(2) 2 1 Copyright – Michael D. Fayer, 2007 Both + and - functions are eigenfunctions of permutation operator. Symmetric function Antisymmetric function eigenvalue +1 eigenvalue -1 All wavefunctions for a system containing two or more identical particles are either symmetric or antisymmetric with respect to exchange of a pair of particle labels. Many electron wavefunctions must be eigenfunctions of the permutation operator. Copyright – Michael D. Fayer, 2007 Inclusion of electron spin in He atom problem Two spin 1/2 particles In m1m2 representation 4 possible states. (1) (2) (1) (2) (1) (2) (1) (2) The two functions, (1) (2) and (2) (1) are neither symmetric nor antisymmetric. m1m2 representation not proper representation for two (or more) electron system. Copyright – Michael D. Fayer, 2007 Transform into jm representation In jm representation (1) ( 2) 11 1 (1) ( 2) (1) ( 2) 2 (1) ( 2) 10 1 (1) ( 2) (1) ( 2) 2 symmetric spin = 1 1 1 00 antisymmetric spin = 0 Copyright – Michael D. Fayer, 2007 Eight spatial functions. H independent of spin. Each of 8 orbital functions can be multiplied by the 4 spin functions. 32 total (spin×orbital) functions. 1s(1)2 s(2) (1) (2) 2 s(1)1s(2) (1) (2) 1s(1)2 p x (2) (1) (2) 1s(1)2 s(2) 1 (1) (2) (1) (2) 2 Copyright – Michael D. Fayer, 2007 Secular determinant looks like 8 8 =0 Block diagonal same as before except the correct zeroth order functions are obtained by multiplying each of the previous spatial functions by the four spin functions. Example 1s2s orbitals Copyright – Michael D. Fayer, 2007 Totally Symmetric 1 1s(1)2 s(2) 2 s(1)1s(2) 1 2 (1-3 spat. & spin sym. 2 4 spat. & spin antisym.) 1 1 1s(1)2 s(2) 2 s(1)1s(2) 1 2 1 2 2 2 1 1s(1)2 s(2) 2 s(1)1s(2) 1 2 2 1 1 1 s (1)2 s (2) 2 s (1)1 s (2) 1 2 1 2 2 2 Totally Antisymmetric 1 (5-7 spat. antisym; spin sym. 1s(1)2 s(2) 2 s(1)1s(2) 1 2 2 8 spat. sym.; spin antisym.) 1 1 1 s (1)2 s (2) 2 s (1)1 s (2) 1 2 1 2 2 2 1 1s(1)2 s(2) 2 s(1)1s(2) 1 2 2 1 1 1s(1)2 s(2) 2 s(1)1s(2) 1 2 1 2 2 2 Copyright – Michael D. Fayer, 2007 Energy Level Diagram Divided according to totally symmetric or totally antisymmetric. Totally Symmetric Totally Antisymmetric 1s2p 1s2p 1P 1s2p 1s2p 3P 1s2s 1s2s 1s2s 1s2s 1S 1s2 1s2 1S symmetric spin function 3S antisymmetric spin function No perturbation can mix symmetric and antisymmetric states (H symmetric). Copyright – Michael D. Fayer, 2007 Which functions occur in nature? Totally symmetric or totally antisymmetric Answer with experiments. All experiments States occuring in nature are always Totally Antisymmetric (Example - ground state of He atoms not paramagnetic, spins paired.) Copyright – Michael D. Fayer, 2007 Assume: The wavefunction representing an actual state of a system containing two or more electrons must be totally antisymmetric in the coordinates of the electrons; i.e., on interchanging the coordinates of any two electrons the wavefunction must change sign. Q.M. statement of the Pauli Exclusion Principle Copyright – Michael D. Fayer, 2007 To see equivalence of Antisymmetrization and Pauli Principle Antisymmetric functions can be written as determinants. A(1) represents an orbital×spin function for one electron, for example, 1s(1)(1), and B, C, … N are others, then A(1) A(2) B(1) B(2) N (1) N (2) A( N ) B( N ) N(N ) Totally antisymmetric because interchange of any two rows changes the sign of the determinant. Copyright – Michael D. Fayer, 2007 Example - He ground state 1s(1) (1) 1s(1) (no bar means α spin) 1s(1) (1) 1s(1) (bar means spin) 1s(1)1s(1) 1s(1)1s(2) 1s(2)1s(1) 1s(2) 1s(2) 1s(1) (1)1s(2) (2) 1s(2) (2) 1s(1) (1) 1s(1)1s(2) 1 (2) (1) 2 Correct antisymmetric ground state function. Copyright – Michael D. Fayer, 2007 Another important property of determinants Two Columns of Determinant Equal Determinant Vanishes Pauli Principle For a given one electron orbital there are only two possible orbital×spin functions, i. e., those obtained by multiplying the orbital function by or spin functions. Thus, no more than two electrons can occupy the same orbital in an atom, and the two must have their spins opposed, that is, no two electrons can have the same values of all four quantum numbers n, , m, ms otherwise two columns will be equal and the determinant, the wavefunction, vanishes. Copyright – Michael D. Fayer, 2007 Example - He ground state with both spins 1s(1) 1s(1) 1s(2) 1s(2) 1s(1) 1s(1) 1s(1) (1)1s(2) (2) 1s(2) (2)1s(1) (1) 0 1s(2) 1s(2) Requirement of antisymmetric wavefunctions is the equivalent of the Pauli Principle. Copyright – Michael D. Fayer, 2007 Singlet and Triplet States Totally Antisymmetric symmetric spin function 1s2p 1P 1s2p 3P 1s2s 1s2s 1S 1s2 1S term symbols 3S antisymmetric spin function singlet states, sym. orbital function × (single) antisym. spin function. triplet states, antisym. orbital function × (three) sym. spin functions. Copyright – Michael D. Fayer, 2007 For same orbital configuration Triplet States lower in energy than singlet states because of electron correlation. Triplet sym. spin. Therefore, antisym. orbital (1) (2) 1 T [1s(1)2 s(2) 2 s(1)1s(2)] ( (1) (2) (2) (1)) 2 (1) (2) For example: Singlet 11 10 1 1 antisym. spin. Therefore, sym. orbital S [1s(1)2 s(2) 2 s(1)1s(2)] 1 ( (1) (2) (2) (1)) 2 00 Copyright – Michael D. Fayer, 2007 Singlet - 2 electrons can be at the same place. Consider a point with coordinates, q. S [1s(1)2s(2) 2s(1)1s(2)] S [1s(q)2s(q) 2s(q)1s(q)] S 2[1s(q)2s(q)] Correlation Diagram Fix electron 1. Plot prob. of finding 2. (1) Prob. of finding electron (2) at r given electron (1) is at q. D q r (schematic illustration) Copyright – Michael D. Fayer, 2007 Triplet - 2 electrons cannot be at the same place. 2 electrons have node for being in same place. Consider a point with coordinates, q (any q). T [1s(1)2 s(2) 2 s(1)1s(2)] T [1s(q )2 s(q ) 2 s(q )1s(q )] Correlation Diagram Fix electron 1. Plot prob. of finding 2. Prob. of finding electron (2) at r given electron (1) is at q. (1) T 0 (1) D D q r r q (schematic illustrations) Copyright – Michael D. Fayer, 2007 In triplet state Electrons are anti-correlated. Reduces electron-electron repulsion. Lowers energy below singlet state of same orbital configuration. Copyright – Michael D. Fayer, 2007







![6) cobalt [Ar] 4s 2 3d 7](http://s2.studylib.net/store/data/009918562_1-1950b3428f2f6bf78209e86f923b4abf-300x300.png)