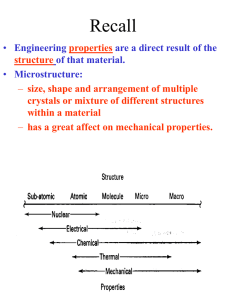
Structure of Solids
advertisement
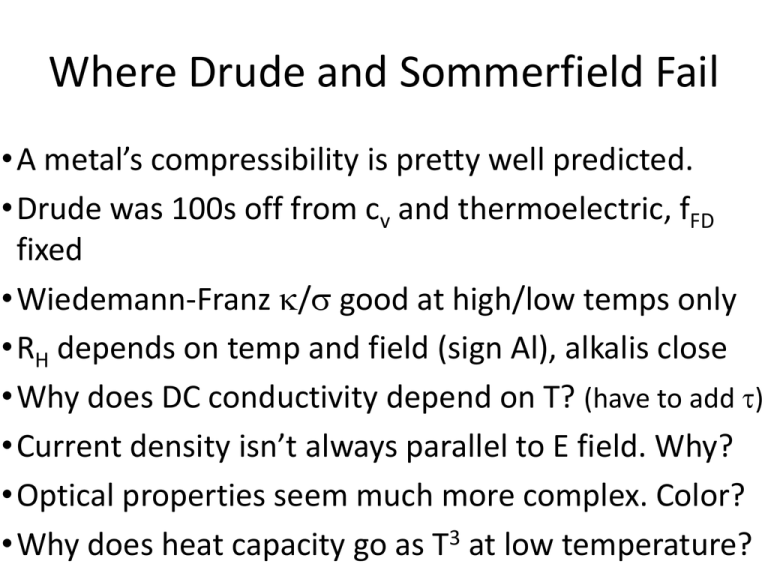
Where Drude and Sommerfield Fail
•A metal’s compressibility is pretty well predicted.
•Drude was 100s off from cv and thermoelectric, fFD
fixed
•Wiedemann-Franz / good at high/low temps only
•RH depends on temp and field (sign Al), alkalis close
•Why does DC conductivity depend on T? (have to add )
•Current density isn’t always parallel to E field. Why?
•Optical properties seem much more complex. Color?
•Why does heat capacity go as T3 at low temperature?
Fundamental Questions Remaining
•What determines the number of conduction
electrons per atom? Some elements (like iron)
have multiple possible valences.
•Why aren’t boron, bismuth and antimony good
[He] 2s 2p
conductors?
2
1
[Kr] 4d10 5s2 5p3
[Xe] 4f14 5d10 6s2 6p3
Limitations of the Drude Model—and Beyond
The Drude model, augmented by quantum mechanics, was
extremely successful in accounting for many of the properties of
metals.
Some flawed assumptions behind the FEG model:
1. The free-electron approximation
The positive ions act only as scattering centers and is
assumed to have no effect on the motion of electrons
between collisions.
2. The independent electron approximation
Interactions between electrons are ignored.
Considerable progress comes from abandoning only the freeelectron approximation in order to take into account the effect
of the lattice on the conduction electrons.
What is crystallography?
The branch of science that deals with the geometric
description of crystals and their internal arrangement.
Platinum
Platinum surface
(scanning tunneling microscope)
Crystal lattice and
structure of Platinum
Structure of Solids
Objectives
By the end of this section you should be able to:
• Use correct notation for directions/planes/families
• Find the distance between planes (when angles 90)
• Identify a unit cell in a symmetrical pattern
• Identify a crystal structure
• Define cubic, tetragonal, orthorhombic and
hexagonal unit cell shapes
Crystal Direction Notation
• Choose one lattice point on the line as an origin
(point O). Choice of origin is completely
arbitrary, since every lattice point is identical.
• Then choose the lattice vector joining O to any
point on the line, say point T. This vector can be
written as;
R = N1 a1 + N2 a2 + N3 a3
• a1, a2, a3 often written as a, b, c or even x, y, z
• To distinguish a lattice direction from a lattice
point (x,y,z), the triplet is enclosed in square
brackets and use no comas. Example: [n1n2n3]
• [n1n2n3] is the smallest integer of the same
relative ratios. Example: [222] would not be
used instead of [111].
• Negative directions can be written as [n1n2 n3 ]
Figure shows
[111] direction
Also sometimes
[-1-1-1]
Group: Determine the crystal directions
X=1,Y=0,Z=0
[1 0 0]
X = -1 , Y = -1 , Z = 0
[110]
[210]
X=1,Y=½,Z=0
[1 ½ 0]
[2 1 0]
X=½ ,Y=½,Z=1
[½ ½ 1]
[1 1 2]
Group: Determine the Crystal Direction
Now let’s do one that’s a little harder.
We can move vectors to the origin as long
as don’t change direction or magnitude.
X =-1 , Y = 1 , Z = -1/6
[-1 1 -1/6]
[6 6 1]
Crystal Planes
In Chapter 5, but useful to know now.
• Within a crystal lattice it is possible to identify sets of
equally spaced parallel planes, called lattice planes.
• The density of lattice points on each plane of a set is
the same.
A couple sets of
planes in a
2D lattice.
b
b
a
a
Why are planes in a lattice important?
(A) Determining crystal structure
* Diffraction methods measure the distance between parallel lattice planes of
atoms to determine the lattice parameters, etc.
(B) Plastic deformation
* Plastic deformation in metals occurs by the slip of atoms past each other.
* This slip tends to occur preferentially along specific crystal-dependent planes.
(C) Transport Properties
* In certain materials, atomic structure in some planes causes the transport of
electrons and/or heat to be particularly rapid in that plane, and relatively slow
not in the plane.
• Example: Graphite: heat conduction is more in sp2-bonded plane.
Miller Indices (h k l )
Miller Indices are a vector representation for the orientation of an a
plane in a crystal lattice and are defined as the reciprocals of the
fractional intercepts which the plane makes with the
crystallographic axes.
To determine Miller indices of a plane, take the following steps:
1) Determine the intercepts of the plane along each of the three
crystallographic directions
2) Take the reciprocals of the intercepts
3) If fractions result, multiply each by the denominator of the
smallest fraction
(multiply again if needed)
Example-1
Axis
X
Y
Z
Intercept
points
1
∞
∞
Reciprocals
Smallest
Ratio
(1,0,0)
1/1 1/ ∞ 1/ ∞
1
Miller İndices
Crystal Structure
0
0
(100)
12
Example-2
(0,1,0)
Axis
X
Y
Z
Intercept
points
1
1
∞
Reciprocals
1/1
Smallest
Ratio
1
Miller İndices
1/1 1/ ∞
1
0
(110)
(1,0,0)
Crystal Structure
13
Example-3
(0,0,1)
(0,1,0)
(1,0,0)
Axis
X
Y
Z
Intercept
points
1
1
1
Reciprocals
1/1
1/1
1/1
Smallest
Ratio
1
1
1
Miller İndices
Crystal Structure
(111)
14
Example-4
Axis
X
Y
Z
Intercept
points
1/2
1
∞
Reciprocals
(0,1,0)
(1/2, 0, 0)
Smallest
Ratio
1/(½) 1/1 1/ ∞
2
Miller İndices
Crystal Structure
1
0
(210)
15
Group: Example-5
Axis
a
b
c
Intercept
points
1
∞
½
Reciprocals
1/1
1/ ∞
1/(½)
Smallest
Ratio
1
0
2
Miller İndices
Note change
of axis
orientation
(102)
Can always shift the plane
(note doesn’t make a difference)
Group: Example-6
Yes, I know it’s difficult to visualize.
That’s actually part of the point of
doing this one.
Axis
a
b
c
Intercept
points
-1
∞
½
Reciprocals
1/-1
1/ ∞
1/(½)
Smallest
Ratio
-1
0
2
Miller İndices
(102)
(102)
What are the Miller Indices (h k l) of this
plane and the direction perpendicular to it?
[2,3,3]
2
c
a
Plane intercepts axes at
Reciprocal numbers are:
3a , 2b , 2c
1 1 1
, ,
3 2 2
Indices of the plane (Miller): (2 3 3)
b
2
Indices of the direction: [2 3 3]
3
Miller indices still apply for a non-cubic system
(even if angles are not at 90 degrees)
If you do have 90 degree angles, use this formula
for distance between planes
Miller Indices (h
k l ), Lattice directions (a, b, c)=(x,y,z)
What is the distance between the (111)
planes on a cubic lattice of lattice
parameter a?
Find the distance between (1 2 3) in a cubic lattice?
Indices of a Family or Form
Sometimes several nonparallel planes may be equivalent by
virtue of symmetry, in which case it is convenient to lump all
these planes in the same Miller Indices, but with curly brackets.
{100} (100), (010), (001), (0 1 0), (00 1 ), ( 1 00)
{111} (111), (11 1 ), (1 1 1), ( 1 11), ( 1 1 1 ), ( 1 1 1), ( 1 1 1 ), (1 1 1 )
Thus indices {h,k,l} represent all the planes equivalent to the
plane (hkl) through rotational symmetry.
Similarly, families of crystallographic directions are written as:
100 [100], [010], [001], [0 1 0], [00 1], [1 00]
Could the
centers of both
Na and Cl be
lattice points at
the same time?
• Crystal Lattice = an infinite array of points in space
• Each lattice point has identical surroundings.
• Arrays are arranged exactly in a periodic manner.
Crystal Structure =Lattice +Basis
• Crystal structure can be obtained by attaching atoms, groups of
atoms or molecules, which are called the basis (AKA motif) to
the lattice sides of the lattice point.
AKA means “also known as”
Crystal structure
• Don't mix up atoms
with lattice points!
• Lattice points are
infinitesimal points
in space
• Atoms can lie at
positions
other
than lattice points
Crystal Structure = Crystal Lattice
Crystal Structure
+ Basis
24
Translational Lattice Vectors – 2D
A Bravais lattice is a set of points such that
a translation from any point in the lattice
by a vector;
R = n1 a1 + n2 a2
P
locates an exactly equivalent point, i.e. a
point with the same environment. This is
translational symmetry.
a2
A a1
Point D (n1, n2) = (0,2)
Point F (n1, n2) = (0,-1)
Point P (n1, n2) = (3,2)
The vectors a1 and a2 are known as lattice
vectors and (n1, n2) is a pair of integers
whose values depend on the lattice point.
What are the lattice points (integers) for
points D, F and P, where point A is the
origin?
Unit Cell in 2D
• The smallest component of the crystal (group of atoms, ions or
molecules), which when stacked
together with pure
translational repetition reproduces the whole crystal.
S
The choice of
unit cell
is not unique.
S
b
S
S
a
26
2D Unit Cell example -(NaCl)
Can the box be a unit cell?
We define lattice points ; these are points with identical
environments
Is this the minimum unit cell size?
Crystal Structure
28
Choice of origin is arbitrary - lattice points need not be
atoms - but unit cell size should always be the same.
Crystal Structure
29
This is also a unit cell it doesn’t matter if you start from Na or Cl
Crystal Structure
30
- or if you don’t start from an atom
Crystal Structure
31
Bravais Lattices in 2D
Special case
where
angles go
to 90
Special case
where
point
halfway
a=b
a=b
In 2D there are five ways
to order atoms in a lattice
Primitive unit cell: contains only one
atom (but 4 points?)
Are the dotted lattices primitive?
Non-primitive unit cells sometimes
useful if orthogonal coordinate
system can be used
Why can't the blue triangle
be a unit cell?
Crystal Structure
33
Lattice Vectors – 3D
(same as the directions we already discussed)
A three dimensional crystal is described
by 3 fundamental translation vectors a1,
a2 and a3.
R = n1 a1 + n2 a2 + n3 a3 (book)
or
r = n1 a + n2 b + n3 c (figure)
Remember any direction [n1 n2 n3] is
perpendicular to the plane (n1 n2 n3).
Sometimes people will use [h k l] instead of n’s for direction too.