cat june2006 sat crowley
advertisement

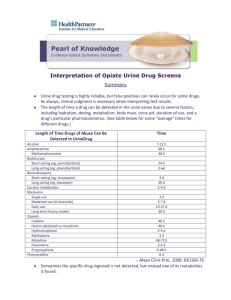
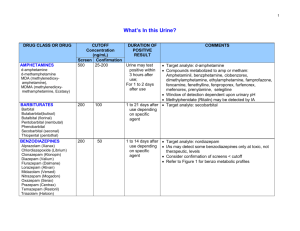
A Practical Method for the Solid Phase Extraction and GC/MS Analysis of 7 Opiates in Urine Paul Crowley Ventura Co. Sheriff’s Forensic Sciences Laboratory Thanks to: Debra Mittelbrun Co-developer Sarah Christiansen, Student Intern References Broussard LA, et al. “Simultaneous identification and quantitation of codeine, morphine, hydrocodone, and hydromorphone in urine as trimethyl and oxime derivatives by gas chromatography-mass spectrometry”. Clin Chem 43:6 1997 pp.1029-1032 Metherall R. “GC-MS Confirmation of Codeine, Morphine, 6-Acetylmorphine, Hydrocodone, Hydromorphone, Oxycodone, and Oxymorphone in Urine”. J Anal Tox. 23 May/June 1999 pp.177-186 Why are we concerned with the confirmation Hydrocodone/ Hydromorphone and Oxycodone/Oxymorphone? Prolific and widely abused. Illegal to possess w/o a prescription. 23152 V.C. (DUI) Often detected in addition to codeine, morphine, and monoacetylmorphine in 11550 H.S. (under the influence/controlled substance) cases. Statistics Screens Total Screens for 01/0512/05: 4133 Opiates Positives: 580 Oxycodone Positives: 27 *started screening for oxy 06/05. Confirmations % Codeine: 322 55.5 Morphine: 434 74.8 MAM: 273 47.0 Hydrocodone: 130 22.0 Hydromorph: 63 10.8 Prior Analysis Scheme Screen for opiates by immunoassay. Confirmation for free codeine, morphine, and monoacetylmorphine by GC/MS. Re-confirm with a second GC/MS method when hydrocodone, and hydromorphone are detected as interferents in the first confirmation. Challenges 2 confirmations if hydrocodone and hydromorphone are suspected. Poor cross-reactivity for oxycodone with opiates immunoassay. No SIMS method for new oxycodone immunoassay (neutral/basic liquid-liquid extraction and GC/MS scan mode). Less than optimal chromatography/instrument response for morphine with old derivatizing scheme (1% Hydroxylamine in pyridine and MtBSTFA). Goals Incorporate all 7 opiates into a single SIMS method. Improve chromatography/instrument response for morphine. LOD low enough for detection of free opiates. (no hydrolysis) Keep run time reasonable. (< 20 min./inj.) Instrumentation Agilent 6890/5973N GC/MS SIM mode/Chemstation J & W Scientific DB-1 capillary column (12 m x 0.20 mm x 0.33 um) Equipment/Materials Cerex System 48 Pressure Processor (Other extractors will work, but must be compatible with 6cc SPE tubes.) SPEware Trace-B Polymer SPE columns (hydrophilic polymer/cation exchanger) Good recovery for all analytes (including morphine). Consistent flow rates/no channeling. Sample Preparation To 2 mL urine add 50 uL of 10 ug/mL dueterated internal standard. (Cod, mor, mam, and oxc – d6) (Hyc, hym, and oxm – d3) Buffer to pH~5 with 2 mL Na Acetate buffer. Extraction Load extractor with SPE columns and transfer samples directly to the columns. *No column pretreatment required. Allow samples to soak on column for 5 minutes. (critical step) Extract at 1 mL/minute. Wash 1 mL H2O 1 mL 0.1M HCl 1 mL MeOH 1 mL ethyl acetate (Dry for 2 min.) *All wash steps were done at 1 mL/min. Elute 2 mL 3% Ammonium Hydroxide/EtAc • 3% more consistent recovery than 2% soln. • Morphine most susceptible to poor recovery. *Avoid* Old NH4OH (> 30 days). Mix well. Derivatization Dry eluant under N2 at 37oC. (No need to transfer before drying/reconstituting) Reconstitute with 50 uL of a 1% Hydroxylamine in Pyridine soln and incubate at 45oC for 30 minutes. Add 50 uL of MSTFA w/1% TMCS and incubate at 65oC for additional 20 min. Why 2-step process ? Hydroxylamine in pyridine soln converts ketoopiates to oximes prior to MSTFA derivatization. • Eliminates codeine and morphine interferents. • Increases silyl derivative efficiency. • Reduces isomeric derivatiztion products. Derivatization Procedures that did not work. Aqueous methoxyamine soln added before sample extraction. Incomplete conversion of keto-opiates to oximes. (Produced isomers) BSTFA not as efficient as MSTFA. Over derivatizing. Heating at 75oC or higher also gave rise to isomeric products. Instrument Conditions DB-1 (12 m) Constant flow (0.5 mL/min) 1.0 uL injection volume/splitless Oven conditions: 100oC for 1 min., ramp to 270oC at 20oC/min., hold 4 min., ramp 40oC/min. to 300oC, hold 0.75 min. (Total run time 15.0 min.) Data Acquisition Elution order Codeine Morphine Hydrocodone MAM Hydromorphone Oxycodone Oxymorphone Target, Qualifier Ions, and R.T.s NIDA Protocol Analyte: 1 quant ion, 2 qualifier ions ISTD: 1 quant, 1 qualifier Analyte/ISTD Codeine Codeine-d6 Morphine Morphine-d6 6-MAM 6-MAM-d6 Hydrocodone Hydorcodone-d3 Hydromorphone Hydromorph-d3 Oxycodone Oxycodone-d6 Oxymorphone Oxymorphone-d3 Quant. Q1 Q2 371 377 429 435 399 405 297 300 355 358 474 480 532 535 343 356 349 414 401 420 400 340 406 386 371 389 444 429 447 385 459 391 517 533 520 RT(min) 8.2 8.5 8.8 8.7 8.9 9.2 9.4 *(12 m column) Acquisition Parameters SIM Analysis Mode/Chemstation 5 Groups (Hyc, MAM, Hym in same group) *Adjust dwell time to 15 or 20 to improve chromatography. Curve and Acceptance Criteria 3 point curve (40 ng/mL, 100 ng/mL and 500 ng/mL) Quantitated by linear regression analysis, forced through zero. Peak well resolved from baseline and Gaussian in shape. I.R. +/- 20% and R.T. 1%. Study Criteria Precision and Accuracy LOD/Sensitivity Linearity Carryover Interference Correlation Precision and Accuracy Prepared samples at 3 levels. (40 ng/mL, 1270 ng/mL, and 2500 ng/mL) *MAM at two levels 6 and 12 ng/mL. Analyzed samples in triplicate, 2 times/day for 10 days. (NCCLS/EP-10A) C.V.’s for all analytes < 10%. Bias at all levels was < 20%. *Oxymorphone out at conc. > 1000 ng/ml. P & A Data Analyte n Codeine 54 Level Low Mid High AVE 43.4 1379 2826 STDev. C.V. (%) Bias(%) 3.54 8.2 -8.5 32.5 2.3 -8.5 45.5 1.6 13.0 Morphine 28 Low Mid High 43.0 1477 3017 2.53 33.5 91.5 5.8 2.2 3.0 7.5 16.3 20.6 Hydroc 54 Low Mid High 40.5 1217 2307 2.56 31.1 35.3 6.3 2.5 1.5 1.3 -4.2 -7.7 MAM 54 Low Mid High N/A 5.54 10.5 N/A 0.18 0.25 N/A 3.2 2.4 N/A -11.4 -16.0 P & A Cont’d Analyte n Hydromorphone 54 Level Low Mid High AVE 41.6 1480 2413 STDev. 1.6 28.2 103 C.V.(%) 3.9 1.91 3.83 Bias(%) 3.9 16.5 -3.5 Oxycodone 54 Low Mid High 40.8 1359 2794 3.9 31.9 6.5 9.5 2.3 1.6 2.0 7.0 11.8 Oxymorphone 54 Low Mid High 48.0 1200 1922 3.59 20.0 80.9 7.49 1.66 4.21 20.0 -5.5 -23.1* L.O.D.’s Sets of 5 replicates at each level (40, 30, 20, 10, and 5 ng/mL). cod: 30 mor: 20 mam: 6 hyc: 20 hym: 20 oxc: 20 oxm: 20 • Goal < 40 ng/mL – 6 ng/mL for MAM. Linearity With the exception of Oxymorphone all analytes demonstrated linearity out to 2500 ng/mL. ( < 20% target conc.) Oxymorphone within 20% of target conc. up to 1000 ng/mL. Bias was > than 20% and was directly related to concentration. Carryover Did experience carryover at concentrations > 2500 ng/mL. Most significant for codeine, followed by oxymorphone and morphine. (Might be a problem for total opiates) Interference Study Two Groups of Compounds: 1.) Possible interferents 2.) Often found in with opiates. Group 1 Methadone Cis-tramadol Naloxone Meperidine Natrexone Papverine Nalorphine Buprenorph. Norcodeine Dextrometh. Normorphine Thebaine Fentanyl Levorphanol Tramadol Propox. Norpropox. Group 2 Amphetamine Oxaz. Methamp Loraz. MDMA Fluraz. MDA Alpraz. Cocaine Temaz. B.E. Diaz. Nordiaz *No interference was observed from compounds from either group. Correlation Study 40 specimens Ante mortem and post mortem All results correlated. (no false positives or negatives) Hydromorphone demonstrated expected loss in concentration due to no hydrolysis. No way to correlate oxycodone and oxymorphone. Final Analysis Suitable P & A, LOD, Linearity, and Carryover characteristics for the qualitative GC/MS confirmation of codeine, morphine, MAM, hydrocodone, hydromorphone, oxycodone, and oxymorphone urine. Good for ante mortem and post mortem urine. LOD’s are compatible for analysis without hydrolysis. Extraction provided good analyte recovery. Derivatization method yielded suitable chromatography. (especially morphine) Thank You for your time. Any questions? References 1.) Broussard LA, Presley LC, Pittman T, Clouette R, and Wimbish GH. Simultaneous identification and quantitation of codeine, morphine, hydrocodone, and hydromorphone in urine as trimethylsilyl and oxime derivatives by gas chromatography-mass spectrometry. Clinical Chem 43:6 1997 pp.1029-1032. 2.) Meatherall R. GC-MS Confirmation of Codeine, Morphine, 6-Acetylmorphine, Hydrocodone, Hydromorphone, Oxycodone, and Oxymorphone in Urine. J Anal Tox Vol. 23 May/June 1999 pp.177-186. 3.) Meatherall R. GC-MS Confirmation of Codeine, Morphine, 6-Acetylmorphine, Hydrocodone, Hydromorphone, Oxycodone, and Oxymorphone in Blood. J Anal Tox Vol. 29 July/August 2005 pp.301-308. 4.) Smith ML, Hughes RO, Levine B, Dickerson S, Darwin WD, and Cone EJ. Forensic Drug Testing for Opiates. VI. Urine Testing for Hydromorphone, Hydrocodone, Oxymorphone, and Oxycodone with Commercial Opiate Immunoassays and Gas Chromatography-Mass Spectrometry. J Anal Tox Vol. 19 January/February 1995 pp.18-26. Reference’s Cont’d Moore KA, Addison J, Levine B, and Smialek JE. Applicability of Opiate Cutoffs to Opiate Intoxication Cases. “Letter to the Editor” J Anal Tox Vol. 25 October 2001 pp.657658. 5.) Trihn A, Marlatt M, and Bell DS. Controlling SPE Selectivity through pH and Organic Modifier Manipulation. “TheReporter” Sigma-Aldrich publication pp.8-9. 6.) 7.) Derivatization Reagents. “Analytix” Sigma-Aldrich publication. March 2002 pp.1-7 8.) Buddha DP, Shimomura ET, and Smith ML. A Practical Approach to Determine Cutoff Concentrations for Opiate Testing with Simultaneous Detection of Codeine, Morphine, and 6-Acetylmorphine in Urine. Clinical Chem 45 1999 pp.510-519. Moore KA, Addison J, Levine B, and Smialek JE. Applicability of Opiate Cutoffs to Opiate Intoxication Cases. “Letter to the Editor” J Anal Tox Vol. 25 October 2001 pp.657658. 9.) 10.) National Committee for Clinical Laboratory Standards. Preliminary Evaluation of Quantitative Clinical Laboratory Methods; Approved Guideline. EP10-A Vol. 18 No. 6 1998.