The Atmosphere

The Atmosphere
Chapter 11
11.1
Atmospheric Composition
About 99% of the atmosphere is composed of nitrogen and oxygen (78% nitrogen, 21% oxygen)
– The remaining 1% consists of small amounts of argon, hydrogen, carbon dioxide, water vapor, & other gases.
Key Atmospheric Gases
The levels of CO2 and water vapor vary constantly.
– The level of these gases are critical because they play an important role in regulating the amount of energy the atmosphere absorbs.
Ozone
Ozone (O3) is important because it absorbs
UV radiation from the Sun thus warming the layer.
Structure of the Atmosphere
The atmosphere is divided into five layers:
– Troposphere
The lowest layer is where most of the weather occurs.
– Stratosphere
Contains Ozone layer
– Mesosphere
Meteors burn up, coldest layer
– Thermosphere
The aurora caused by
- Exosphere
Outermost layer particles from the Sun i nteracting with Earth’s atm.
– Ionosphere
Charged particles reflect radio signals
Structure of the Atmosphere
Radiation
Radiation is the transfer of energy through space by visible light, UV radiation, and other forms of electromagnetic waves
– ~30% of incoming solar radiation is reflected into space by Earth’s surface
– ~20% is absorbed by the atmosphere itself
Only 50% of incoming solar radiation is absorbed by
Earth’s surface
Radiation
The rate of absorption for any area varies depending on the physical characteristics of the area and the amount of solar radiation it receives.
– *Land heats and cools faster than water
– Darker objects absorb energy faster than lighter ones
Radiation
Conduction
Energy is transferred throughout the atmosphere by the process of conduction, convection & radiation.
Conduction is the transfer of energy that occurs when molecules collide.
– Energy is transferred from the particles of air near Earth’s surface to the particles of air in the lowest layer of the atmosphere.
Convection
Convection is the transfer of energy by the flow of a heated substance.
– Pockets of air near Earth’s surface are heated, become less dense than the surrounding air & rise.
– As the warm air rises, it expands and starts to cool.
– When it cools below the temperature of the surrounding air, it increases density and sinks.
Convection Currents
Convection
Conduction, Convection,
Radiation
Chapter 11.1 Quiz
1. Name the 5 layers of the atmosphere in order from farthest to closest to Earth’s surface.
2. What is significant about the ionosphere?
3. What are the 2 main gases in our atmosphere and what is the percent they make up of the atmosphere?
4. ____________ is the transfer of energy that occurs when molecules collide.
5. The level of __________ & __________ are critical because they play an important role in regulating the amount of energy the atmosphere absorbs.
11.2
State of the Atmosphere
Temperature is a measurement of how rapidly or slowly molecules move around.
– fast moving molecules = higher temp.
– Slow moving molecules = lower temp.
Heat is the transfer of energy that occurs because of a difference in temperature between substances.
– Heat flows from area of high temp. to area of low temp.
Dew Point
The dew point is the temperature to which air must be cooled at constant pressure to reach saturation.
– Saturation is the point at which the air holds as much water vapor as it possibly can.
Condensation occurs when matter changes state from a gas to a liquid.
Moisture in the Atmosphere
When the air is holding as much moisture as it can, the air is saturated .
– The air’s ability to hold water vapor depends upon the temperature.
– The warmer the air, the more moisture the air can hold.
The dew point is the temperature to which the air must be cooled to become saturated.
– If the temperature falls below the dew point, condensation occurs as water vapor changes to liquid water.
Vertical Temperature Changes
Dry Adiabatic Lapse Rate: the rate at which unsaturated air (which no heat is added or removed) will cool.
– 10°C for every 1,000m increase in altitude.
Moist Adiabatic Lapse Rate : the rate at which saturated air cools.
– ~4°C/1,000m in very warm air to ~9°C/1,000m in very cold air.
Vertical Temperature Changes
Lifted Condensation Level (LCL): the height at which condensation occurs.
– LCL often corresponds to the base of clouds
– Air above the LCL is saturated and cools slower than air below the LCL.
Air Pressure & Density
Air has mass and exerts pressure on our bodies.
– Atmospheric pressure increases as you near the bottom of the atm because of the greater mass of atm above you.
– Atmospheric pressure decreases with height because there are fewer gas particles exerting pressure.
Air Pressure & Density
The density of air is proportional to the number of particles of air occupying a particular space.
– The density of air increases as you get closer to the bottom of the atmosphere.
– The density of air decreases as you increase elevation.
Pressure-Temperature-Density
Relationship
Temperature is directly proportional to pressure.
– As temp increases/decreases, pressure does too
– As pressure increases/decreases, temperature does too
Pressure-Temperature-Density
Relationship
The relationship between temperature and density is inversely proportional.
– As temperature increases, density decreases
– As temperature decreases, density increases
**Temperature is proportional to the ratio of pressure to density, which decreases with increasing altitude.
Temperature Inversion
A temperature inversion is an increase in temperature with height in an atmospheric layer.
– temp-altitude relationship is inverted
Temperature Inversion
Wind
Cool air = more dense, sinks
Warm air = less dense, rises
– **Air flows from areas of high pressure to areas of low pressure
Wind changes with height in the atm
– Near the surface, wind is disrupted by the friction that results from contact with trees, buildings, etc.
– Farther up from Earth’s surface, air encounters less friction & wind speeds increase
Wind
Relative Humidity
The amount of water vapor in air is referred to as humidity .
The ratio of water vapor in a volume of air relative to how much water vapor that volume of air is capable of holding is called relative humidity .
– RH is expressed as a percentage %
Relative Humidity
Compares how much moisture the air is actually holding with how much moisture it could hold if the air were saturated.
– It is expressed as a percent of saturation.
Air is saturated if it is holding all the moisture it can hold at its present temperature.
Determined with a psychrometer and a relative humidity table.
Relative Humidity
Warm air is capable of holding more moisture than cool air.
– If the temp. of a room increased, the air in the room would be capable of holding more moisture.
If no additional water vapor was added to the air, its relative humidity would decrease.
If more water vapor was added to the air, its relative humidity would increase.
Relative Humidity
11.3
Moisture in the Atmosphere
Cloud Formation
Clouds form when rising air is cooled below its dew point.
Tiny particles called condensation nuclei (small
particles in the air) allow a cloud to form.
Cloud Formation
Clouds can also form when wind encounters a mountain and the air has nowhere to go but up.
– As the air rises it cools and condenses.
– This is method of cloud formation is called orographic lifting .
Cloud Formation
Another method of cloud formation involves the collision of air masses of different temps.
– Cold, more dense air mass will collect near the surface.
– As warm air moves into the area, some of it will warm up the cold air but the bulk will rise over the cold air.
As the warm air cools, the water vapor in it condenses and forms a cloud.
Stability
stability = tendency of air to remain in in its original position (resist rising).
– The rate at which an air mass cools depends on the temperature of the surface beneath the air.
If the air = cooler; it is going to want to sink stable
If the air = warmer; it is going to want to rise unstable
Stability
Latent Heat
As water vapor in the air condenses, heat is released.
It takes energy to change liquid water into gaseous state
– That energy is stored in the water vapor and will not be released into the air until condensation occurs.
The stored energy is called latent heat .
– When condensation occurs, latent heat is released and warms the air.
Types of Clouds
Clouds are classified by the altitude at which they form and by their shape.
Low Clouds
Typically form below 2,000m
– Stratocumulus : gray/whitish patch/sheet that is flattened out and spread horizontally
– Cumulus : detached, generally dense clouds with sharp outlines that develop vertically in the form of rising mounds.
– Stratus : layered gray cloud that covers much or all of the sky in a given area
– Cumulonimbus : thunderstorm cloud, dark at the bottom that produces rain and/or hail.
Cumulus
Low Clouds
Stratus
Cumulonimbus
Stratocumulus
Middle Clouds
Form at heights between 2,000 and 6,000m
– Altocumulus : white/gray patch or sheet layered clouds (resemble fish scales)
– Altostratus : dark but thin veils of clouds that sometimes produce mild precipitation and cover totally or partially the sky. Thin enough to see the sun.
– Nimbostratus : the continuous rain cloud resulting from thickening altostratus. Completely block out the Sun. Dark gray in color; associated with precipitation.
Middle Clouds
Altostratus
Altocumulus
Nimbostratus
High Clouds
Form at heights of 6,000m
– Temps are below freezing, thus made up of ice crystals.
– Cirrus : wispy, indistinct (detached) appearance
– Cirrostratus : transparent, whitish veil clouds with a smooth appearance. continuous layer that covers the sky
– Cirrocumulus : thin, white patch sheet or layered clouds
Cirrus
High Clouds
Cirrostratus
Cirrocumulus
Types of Clouds
Precipitation
When cloud droplets collide, they join together to form a larger droplet in a process called coalescence .
As the process continues the droplet becomes too heavy to be held aloft and falls to earth as precipitation .
– Rain, snow, sleet, and hail
Precipitation
Rain and snow are the most common forms of precipitation.
Drizzle is small raindrops that fall slowly
Sleet is a partially frozen mixture of rain and snow that occurs when the temperature is just above freezing.
Hail is in the form of ice balls, which usually occurs in violent thunderstorms.
– Hailstones begin as snowflakes that start to melt and gather more moisture as they fall.
The Water Cycle
Water moves between Earth’s surface and its atmosphere water cycle
1. Radiation from the Sun causes liquid water to change into a gas evaporation
2. As water vapor rises, it cools and changes back into a liquid condensation
3. Water droplets combine to form larger drops that fall to Earth precipitation
The Water Cycle
Weather
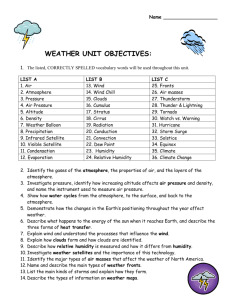
The short-term (a few hours or days) condition of the atmosphere at a given location.
– Temperature, sky conditions, precipitation, atmospheric pressure, humidity, wind speed, and wind direction.
Meteorologists are scientists who study and predict the weather.
Air Temperature
In the daily cycle, temperature is usually lowest in the early morning and warmest at mid-afternoon.
In the season cycle, winters are generally cold, while summers tend to be hot.
Short term factors such as cloud cover and regional weather systems affect temperatures.
– Clouds reduce daytime temperature by reflecting sunlight back into space.
– At night, clouds help hold heat energy to Earth.
Air Temperature
Measured with a thermometer .
– A bulb that contains liquid that expands into a narrow, calibrated neck when it is heated and moves down the neck when the temperature decreases.
When meteorologists record official air temperature, the thermometer is kept in a special weather shelter to protect the instruments from direct sunlight.
Temperature Scales
A temperature of zero on the Fahrenheit scale is the temperature of a mixture of equal parts ice, water, and salt.
The freezing point of water is what sets the zero point on the
Celsius (centigrade) scale.
The point at which all particle motion stops is defined as zero on the Kelvin scale.
Air Pressure
Is caused by the weight of the atmosphere.
Above each square inch of Earth’s surface is a column of air the weighs 14.7 pounds.
Measuring Air Pressure
A barometer is an instrument used to measure air pressure using the dense liquid metal mercury.
Meteorologists measure air pressure in millibars .
Standard sea level pressure is 1013.2 millibars.
On a weather map, isobars connect places that have the same air pressure.
Air Pressure Factors
If air is cooled, it contracts and becomes denser.
– This causes pressure to rise.
If air is heated, it expands and becomes less dense.
– This causes pressure to fall.
Humid air is lighter than dry air.
– This is because water molecules are lighter than the gasses they displace in the air.
Measuring Moisture in the Atmosphere
Meteorologists use a sling psychrometer and a dew-point temperature table to determine the dew point.
– The psychrometer consists of two thermometers mounted side by side which can be swung through the air.
– One thermometer measures the air temperature.
– The bulb of the other thermometer is covered by a wet cloth.
– As the thermometers are swung through the air, evaporated cooling causes the wet-bulb thermometer to register a lower temperature.
– When you subtract the wet-bulb temperature from the drybulb temperature, you can use the dew-point table to determine the dew point.
Psychrometer
The Wind
Wind is heat flow by convection within the atmosphere.
Winds are the result of uneven heating of the Earth’s surface.
– This uneven heating causes differences in air pressure to develop.
The Wind
Winds always blow from areas of high pressure to areas of low pressure.
– Winds blow fastest where the gradient in air pressure is greatest, where the isobars are close together.
Measuring the Wind
To measure the wind, you need to determine both the wind speed and the wind direction.
Wind speed is measured with an anemometer .
– The cups catch the wind, causing it to spin.
Wind direction is indicated by a wind vane, which points into the wind.
The Coriolis Effect
The Earth’s rotation causes winds to curve.
– to the right in the Northern
Hemisphere .
– to the left in the Southern
Hemisphere.
The Coriolis Effect
Winds move in a clockwise outward spiral around high-pressure systems.
Winds move in a counterclockwise inward spiral around low-pressure systems.
The Coriolis Effect
Zones of
Convergence and Divergence
Rising warm, moist air at the center of the low causes winds and air masses to blow into the low-pressure system.
The rising air cools, which causes cloud formation and precipitation.
The descending air turns a high-pressure system into a single mass of cool, dry air that spreads across the surface of Earth.