Measuring Volume
advertisement
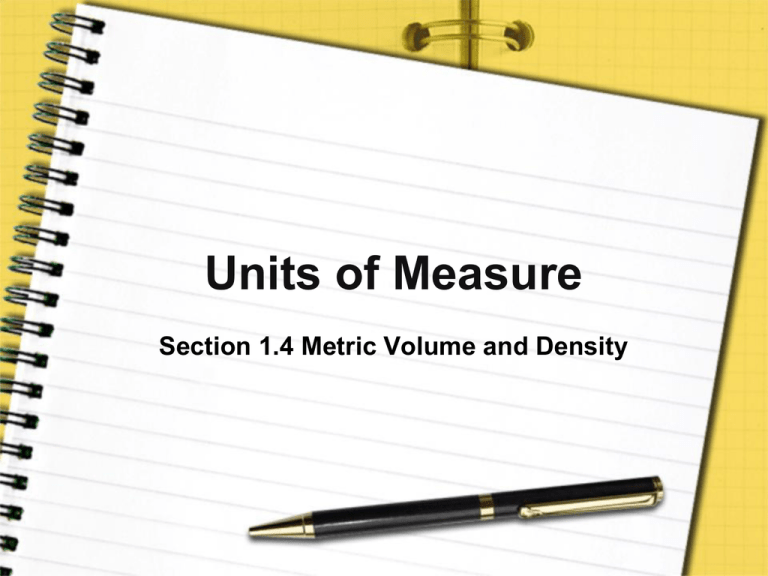
Units of Measure Section 1.4 Metric Volume and Density Pre-View 1.4 • Volume – the amount of space an object occupies • Cubic meter – the amount of volume in a box that is one meter long, one meter wide, and one meter high • Liter – common metric unit used to measure liquid or gas volume • Milliliter – the volume equal to one cubic centimeter (cm3) Pre-View 1.4 • Water displacement – a method used to find the volume of an irregularly shaped object. • Graduated cylinder – piece of equipment that can be used to find the volume of an irregularly shaped object. • Density – the measure of mass per unit of volume; calculated by dividing mass by volume. Measuring Volume • Volume is a measurement of three dimensions and is calculated by multiplying length, width, and height. – For example, the volume of one cubic meter (m3) can be represented by a box that is one meter long, one meter wide, and one meter high. Measuring Volume • Instead of using cubic units like m3 or cm3, volume can also be expressed in liters (L). • One cubic centimeter is equal to one milliliter (mL). • Solid volume is usually given in cubic meters or cubic centimeters, but gas and liquid volumes are commonly given in liters or milliliters. Measuring Volume – Regularly Shaped Objects • Regularly shaped objects are objects that are square, rectangular, cylindrical, coneshaped, or spherical. • By using a ruler to determine one or more of their dimensions, you can then use a formula to determine volume – Example – to determine the volume of a rectangular object, measure each dimension. Then multiply the three dimensions together. Measuring Volume – Regularly Shaped Objects For Example: Using a ruler, John determines his biology textbook is 25 centimeters wide, 30 centimeters long, and 3 centimeters thick. What is the volume of the textbook: 25 cm x 30 cm x 3 cm = 2250 cm3 The textbook’s volume is 2,250 cm3 Measuring Volume – Irregularly Shaped Objects • Many objects are not perfectly rectangular. • Water displacement can be used to obtain the volume of an irregularly shaped object. – First, a known volume of water is recorded. – Then the object is submerged in the water, and a water plus object volume is determined. – The difference in the first volume and the second volume is the volume of the object. Measuring Volume – Irregularly Shaped Objects Example • Dr. Watts, a paleontologist, wants to know the volume of a fossilized bone. • She measures exactly 10 mL of water in a graduated cylinder. • She then drops the bone into the graduated cylinder. • The volume of the water and the bone measures 12.5 mL. What is the volume of the bone? Measuring Volume – Irregularly Shaped Objects Example (continued) 12.5 mL – 10 mL = 2.5 mL The bone has a volume of 2.5 mL, which is the same as 2.5 cm3. SI unit of Volume: cubic meter (m3) • When to use it… – Use cubic meters for large volumes. • Example: – The water volume in an Olympic swimming pool is about 2500 m3 SI unit of Volume: liter (l) • When to use it… – Use instead of quarts or gallons. • Example: – A common size for a large soft drink container is 2 liters SI unit of Volume: cubic centimeter (cm3) or milliliter (ml) • When to use it… – Use cubic centimeters or milliliters for small solid or liquid volumes. • Example: – A small cellular phone is about 90 cm3 – A 20 ounce soft drink bottle holds about 590 ml Measuring Density • Density is mass per unit of volume and is a common value measure in science. – To get an idea of what density is, think about a marble and a small styrofoam ball that are the same size and have the same volume. – The marble will sink in water, but the styrofoam ball will float. Measuring Density – If they are the same volume, why does one sink and the other float? • The answer is density. • The marble is dense and has more mass in the same amount of volume than the styrofoam. • In general, an object that has a density greater than the density of water will sink in water. Measuring Density • An object having a density less than the density of water will float. • Water has a density of one gram per milliliter (1 g/mL) To determine density of an object, you divide the mass of the object by the volume of the object. The resulting units are commonly g/cm3, kg/m3, g/mLor kg/L Measuring Density Example • In the previous example, Dr. Watts found the volume of a fossilized bone to be 2.5 mL. She determined that the mass of the bone is 9 grams. What is the density? Will it float or sink in water?