Solid State Materials Chemistry
advertisement

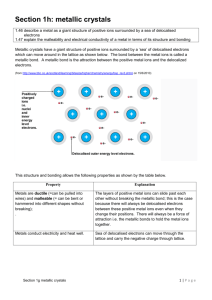
Solid State Materials Chemistry – Walton Revision Hand-Out 1 Classifications of Materials Based on dimensionality 3D Solid Structures Bonded covalently or ionically, in all 3 directions. Examples include SiO2 (Covalent) and NaCl (Ionic). Usually modelled as ball and stick models or tetrahedrons. 2D Solid Structures Strong bonds in two directions (Ionic or Covalent). Weak bonds in the third direction (Hbonds or v.d.w.). Usually occur as stacked layers. 1D Solid Structures Chains of atoms Strong bonds in one direction, weak bonds in two directions. Examples include red phosphorous and SiS2. 0D Solid Structures Strong bonds within molecules Weak bonds between all molecules (v.d.w.) Examples include I2 Examples of practical uses of these compounds are Polymers (1D), Liquid crystals (0D), Biological Materials (3D). Carbon can exist in more than one structure type: Diamond – 3D Solid Structure – sp3 carbons, electronic insulator as all e- used in bonding Graphite – 2D Solid Structure – sp2 carbons, electronic conductor as delocalised π e- can carry charge C60 – 0D Solid Structure Case Study 1 – Rechargeable Batteries Rechargeable batteries contain two crystals separated by a non-aqueous electrolyte. The crystals are made of LiCoO2, a 2D solid Structure composed of layers of interconnected CoO2- and lone Li+ ions. When the battery is being charged the electrons move from one side to another. They are then discharged through the electrolyte in order to release energy. The main issue is that Co is expensive and therefore alternatives are being looked for. The Sodium Chloride Structure The NaCl structure is described as cubic close packed chloride anions with all octahedral sites filled by sodium. Crystallographic Notation: The Lattice A Lattice is an arrangement of points in space such that the lattice looks the same from whichever lattice point you view it from. The lattice describes the translational symmetry of an arrangement of objects. The objects, or basis, may or may not sit on lattice points. In the figure to the right, Na and Cl are the basis. Na Cl Na Cl Na Cl Na Cl Na Crystallographic Notation: The Unit Cell The Unit Cell is the smallest, highest symmetry volume of a structure that allows the three dimensional structure to be constructed in combination with the lattice and purely by translational repeats. In the diagram above, a 2x2 square containing 2 Na atoms and 2 Cl atoms is not the unit cell, however the full 3x3 unit is. The simplest description of the unit cell is given on the right. Atom positions are given as fractions relative to the total size of the unit cell when drawn in 3D and are used for the depth in the 2D projection of the unit cell. Counting of atoms within a unit cell is carried out based on how much of an atom is inside that specific cell. i.e. those on the corner of a cube are included 1⁄8, those on a face 1⁄2. The arrangement of the unit cell and atoms within the unit cell are described using symmetry. These are the same symmetry elements as taught by Dove in 1st year. Symmetry in 3D The unit cell has inherent Symmetry e.g. a cube has 4 C3 axis along each body diagonal. This is shown on the cube on the right. Other symmetry applications as well as proper rotations are possible; mirror planes, improper rotations. Including atoms into the 3D unit cell can induce translational symmetry. For example the pairing of a mirror plane with a translation is known as a “glide plane”. Summary of Crystal Structures To define a 3D infinite structure, the following information is needed: The unit cell shape and lattice parameters The basis (location of unique atoms in the unit cell) The translational symmetry of the 3D structure Cubic Close Packing Also known as face centred cubic, this packing arrangement consists of atoms on the vertices and the centre of the faces of the unit cell. The diagram to the left. The tetrahedral holes are indicated on the diagram to the right. Filling Octahedral Holes The NaCl structure obeys this packing arrangement however the octahedral holes of the Na ions are filled by the Cl ions. Other combinations also exist, for example CdCl2 where only half of the octahedral holes are filled, with sheets of holes being present as shown in the diagram as the hashed blue atoms. CdCl2 exists as sheets of edge shared octahedra. Other more complicated structures also exist, CrCl3 fir example consists of CCP Cl- ions with Cr occupying two thirds of the octahedral holes in every other layer of Cl. In the diagram below the Cl ions are green while the Cr Ions are grey. Filling Tetrahedral Holes In addition to having CCP structures with the octahedral holes filled by other ions, it is also possible to have the much smaller tetrahedral holes filled by other ions. One example of this is the fluorite structure, which contains CCP Calcium with all tetrahedral holes filled by F- ions. ZnS is another example with half of the tetrahedral holes in the CCP Sulphur lattice filled with Zn2+. This is known as the zinc blende structure. The Radius Ratio Rules Co-ordination Number 4 6 8 Geometry Tetrahedral Octahedral Cubic r+/r- Ratio 0.232 – 0.414 0.414 – 0.732 0.732 – 1.000 Possible Structures ZnS (wurtzite or zinc blende) NaCl or TiO2 (rutile) CsCl or CaF2 (fluorite) Electron Density Maps This shows the concentration of electrons within a crystal structure, as expected the lowest density is at the point directly in the centre of the atoms. This can also be deduced from a graph plotting the density against the distance from the nucleus. The graph above shows the electron density with respect to the distance from the Li+ and F- nuclei. The various measures of electronegativity are shown on the diagram. As expected the minima of the graph is at the point directly between the ions. Chemical Bonding As the polarisation of a bond increases, the dimensionality decreases. Highly ionic compounds like CaF2 exist in regular repeating 3D units. However compounds with more covalent character exist in 2D and 0D structures such as HgI2 and SiS2 respectively. Structures of Transition Metal Dioxides Transition metal monoxides, of the formula MO, usually exist in the NaCl structure. Metal dioxides however exist as crystals with the rutile structure. This is formed of a tetragonal unit cell, i.e. a = b ≠ c. An example of the TiO2 structure is shown. Ti is grey, O is red. A plan view of the diagram is shown on the left. Structures of Transition Metal Trioxides Transition metal trioxides exist in a cubic structure, W and Re oxides. On the diagram to the right, the metals are blue and oxygen red. This structure can also be thought of as octahedral units joined by the corners. WO3 however has two possible distinct structures. At room temperature the crystal deviates from the cubic structure to a monoclinic structure, in which the octahedra are slightly offset from each other Structure of Ternary Transition-Metal Oxides These materials have the molecular formula ABO3. They are the same as the cubic structure however there is another atom in the centre of each set of 8 corner joined tetrahedra. This structure is known as perovskite. This consists of a large 12 co-ordinate cation in the centre, A. Surrounded by 6 coordinate metal ion B in the centre of each octahedron. The alternative structure for ternary oxides is the spinel, AB2O4, which is similar to CCP oxide. Half octahedral sites filled by B, one eighth tetrahedral sites filled by A. A = Tetrahedral B = Octahedral Case Study 2 – Capacitors Use BaTiO3 which has the perovskite structure. Its polarity is changed by the temperature. Above 120ᵒC – Ideal perovskite structure. Below 120ᵒC – Distorted perovskite structure. Changes the polarity. This causes spontaneous electric polarisation. This polarisation is reversible by application of an electric field. Hand-Out 2 Polymorphism Polymorphism is the ability of a material to exist in more than one crystal structure. This is altered by the temperature and pressure a material is put under. One example of this is carbon, which undergoes reconstructive phase transitions. These involve the making and breaking of bonds. These transitions are described as reconstructive as the actual structure of the crystal has changed. Bonds must be broken and reformed. For example in the transition from graphite to diamond sp2 carbons must change to form sp3 carbon because of this reconstructive phase transitions have reasonably high energy barriers. Reconstructive phase transitions are irreversible. Silicon dioxide also displays reconstructive phase transitions, for example from four coordinate coesite to six co-ordinate stishovite. SiO2 can undergo displacive phase transitions, these have a negligible energy barrier and do not involve the breaking or formation of any of the bonds. These phase transitions are also very easily reversible. One example of this is the transition from α SiO2 to β SiO2. Displacive phase transitions are characterised by small changes in density. Reconstructive phase transitions have much greater differences. Thermodynamics of Phase Transitions If the value for ΔG at a phase transition is equal to 0, then the phases must coexist, i.e. you are at the Tc for those two phases. Tc is the temperature at which two phases are at equilibrium. A plot of G against T can be used to determine if a transition is reconstructive or displacive. First Order Phase Transitions In this graph there is a definite change in G as the temperature increases. This is indicative of a discontinuity in volume and entropy. This is a reconstructive phase transition Second Order Phase Transitions This graph shows a much more subtle change in G after the critical temperature, this is indicative of a displacive phase transition as there is no large change in volume and hence entropy. Glasses and Amorphous Materials Amorphous materials are structures with a close range order however lack the long range order displayed by a crystal. This can be seen in the radial distribution function of the material. Below is the comparison between amorphous glass and crystalline quartz. The quartz displays a regular order even at long range. In glass this long range order is not present and despite having a very similar structure the radial distribution looks very different to quartz. Network Modifying Ions Because of this amorphous structure, network modifiers can be added to impart mechanical or optical properties not usually displayed by the glass. For example the addition of 15B 2O3 and 5Na2O for every 80SiO2 molecules creates Pyrex which has a much higher melting point and is more structurally stable than normal glass. Another way by which glasses are modified is to add cations which stabilise the negative charge held by any terminal oxygen ions. Case Study 3 – Aluminosilicate Zeolites Replacement of tetrahedral Si in zeolites by Al causes a net negative charge on the framework, this creates the formation of a nanoporous material. These have applications as Ion exchangers for example in water softening tablets. Na2[Al2Si2O6]·nH2O + Ca2+ Ca[Al2Si2O6] + 2Na+ Defects and Non-Stoichiometry Defects occur because ions are only at their ideal lattice positions when T = 0K. Above this thermal energy is changed into kinetic energy and the atoms are displaced from their ideal sites. This gives rise to a large increase in entropy. S = k ln(W) Where W is the number of ways of arranging a defect. This means that defects are entropically favourable and hence occur. Intrinsic Defects – No change in composition Schottky Defects – Pairs of vacant sites in the crystal structure, these are most common in crystals with a 1:1 composition. The charge overall is the same as complementary ions have been removed. Frenkel Defects – The migration of an ion to an interstitial site, these usually occur in polarisable cations however migration of anions is possible. Calculating the number of Intrinsic Defects ns Ne Es 2 kT The derivation of this is given in the notes. As an example for NaCl at room temperature (300 K) the value of ns/N ~ 10-20 hence the number of defects is very small however this number increases with temperature. Extrinsic Defects: Dopants in the Solid This is caused by the addition of a small amount of a new ion which balances the charge left by any single ion vacancies. For example a Ca2+ ion can replace 2 Na+ ions in NaCl with no overall change in structure. Nomenclature for Defects in Solids Defects in solids are given in the form of Kroger-Vink notation. This is usually in the form ECS. E is the symbol for the atom, or V for vacancy C is the net charge of the vacancy o · = one unit of positive net charge o × = zero net charge o ‘ = one unit of negative net charge S is the nature of the site o Element symbol for existing site in crystal o i = interstitial site = Frenkel Defect o s = surface site Examples of Kroger-Vink Notation V’Na = Vacancy in Na site, -1 net charge Ca·Na = Ca2+ ion in place of an Na+, +1 net charge Ag·I = Interstitial Ag+ ion, +1 net charge Cl×Cl = Fully occupied Cl site, 0 net charge Effects of Defects Migration of ions between vacant sites provides a means of carrying charge if a potential is applied to a crystal Vacancies move in the opposite direction to the Ions Migration of ions between interstitial sites can also occur Magnitude of Conductivity σ=nZeμ σ = Conductivity (sm-1) n = Number of charge carriers Z = Charge on charge carriers E = Charge on an electron μ = Mobility of the species Ionic Crystals Solid Electrolytes Liquid Electrolytes Ionic Conductors Electronic Conductors Conductivity 10-16 - 10-2 10-1 - 103 Metals 103 - 107 Semiconductors 10-3 - 104 Insulators < 10-10 Ionic conductors have lower conductivities than electronic conductors as electrons move much faster than ions. Quantifying Electrical Conductivity In this diagram, the parabolic path shown by the points 1, 2 and 3 is the preferred route by which electrons move from one unit cell to the next. This minimises repulsion from the ions on the corner of the octahedra. This motion has an activation barrier and hence a temperature dependence. μ = μ0 exp(-Ea/kT) This is the equation for the mobility of an ion. This can be substituted into the equation above to give: Ea ( 0 )e kT where 0 nZe0 ln( ) ln( 0 ) Ea kT This can then be plotted as a graph of ln(σ) against 1/T which gives a straight line with gradient –Ea/k. If the attempt frequency of a hop is reduced at higher temperature then the following equation is used: ( 0 T )e Ea kT Ea T ( 0 )e kT ln( T ) ln( 0 ) Ea kT Hence ln(σT) is sometimes plotted against 1/T. A linear behaviour is generally seen, however not always. For example in the case of LiI or NaCl the following graph results: At low temperature the number of Schottky defects is low, and therefore conduction is dominated by extrinsic defects in the ionic crystal. At high temperature Schottky defects become significant and conduction is no longer dominated by extrinsic defects. The gradient of the graph has changed as the value for E g is now different. Silver Iodide Conduction can also be altered by phase transitions for example in the case of AgI. At less than 146ᵒC AgI takes a Wurtzite structure, above this temperature it exists in a body centred arrangement of I-. This is an example of a ‘fast-ion’ conductor as in the body centred arrangement it has the conductivity of an ionic liquid ~ 130 Sm-1. Stabilised Zirconia ZrO2 has the cubic fluorite structure. This is an open lattice with frenkel defects. This is then doped with Y3+ into the Zr4+ sites. This causes more oxide ion vacancies in order to balance the charge. This means that oxide ions are free to carry the charge. This doping has an effect on the structure too. As the mol% of Y2O3 is increased, the Lattice parameter increases. Case Study 4 – Oxygen Detection Detectors containing Yttrium Stabilised Zirconium are used to measure the concentration of O2 across a partial pressure potential. A solid oxide ion electrolyte allows the flow of oxide ions and hence current to be generated. Case Study 5 – Solid-Oxide Fuel Cells These cells control the combustion of hydrogen, methanol or hydrocarbons in order to generate electricity which can then be used to power cars or other electrical items. They are a ‘green’ energy supply as they only produce water. The electrolyte in these cells is again YSZ. Metal Oxides and Non-Stoichiometry For metals with variable oxidation sites, vacancies can also be balanced by a change in metal oxidation state. For example FeO actually takes the composition Fe0.87-0.93O. This is known as non-stoichiometry. Debate as to whether this is caused by iron vacancies or excess oxygen. Density measurements give value lower than expected. Therefore there must be iron vacancies. Some Fe2+ must be oxidised to Fe3+. Fe2+(1-3x)Fe3+(2x)O The situation is in fact more complicated than this, there are more vacant octahedral sites than expected. This means that Fe3+ must sit on the interstitial tetrahedral sites. These form something known as the “Koch-Cohen” cluster. Fe2+ remains in Oh sites due to favourable d6 CFSE. Titanium monoxide, TiO1.000, is based on the rock salt structure but has 1/6th of Ti and O missing in order to maintain stoichiometry. The unit cell for this structure is shown on the right. Every second atom along every third diagonal is missing. TiO1.25 also exists, this has a similar structure however all oxygens are present and 1/5th of Ti are missing. In this case some of the remaining Ti is oxidised from TiII to TiIII. TiII0.4TiIII0.4O Uranium dioxide also displays non-stoichiometry. UO2 displays the fluorite structure with 8 co-ordinate U and 4 co-ordinate O. Compounds with formula up to UO2+x (x < 0.25) are also possible due to the partial oxidation of UIV to UV. There is a possible interstitial site for oxide at a point in the very centre of the unit cell i.e. (0.5,0.5,0.5) however this site is shown to be unoccupied by neutron diffraction. The actual site is slightly offset from the centre and causes the displacement of other oxide ions from their ideal sites also. At limiting composition of U4O9 all sites are perfectly ordered again. Tungsten and Rhenium Trioxide display a network of corner and edge shared octahedral units. Both of these metals can be reduced to lower oxidation states and hence oxygen deficient phases are possible. The loss of oxygen causes a distortion to the structure in such that octahedra become edge shared. This gives a series of possible phases: MnO(3n-2) where n = 20, 24, 25, 40 and M = W or Re Case Study 6 – Cerium Dioxide in Catalysis The occurrence of non-stoichiometric oxides is used in catalytic converters to reduce harmful gasses. x CeO2 CeO2-x + O2 2 This released oxygen then reacts with CO and NOx to form N2, H2O and CO2. Hand-Out 3 Classification of Conductors by Band Theory Effect of Ionic Character on Band Gap As the difference in electronegativity of two ions is greater, the p and s bands of electrons become more distinct. This causes localisation of electrons and therefore the band gap increases. For example KBr, ZnSe and GaAs are isoelectronic in every sense. The only difference is their electronegativity. The band gaps in KBr is 7.5 eV whereas the band gap in GaAs, compounds with much more similar electronegativity, is 1.4 eV. Colour and Electronic Conductivity Fine powders of metals and semiconductors are black – all visible wavelengths of light are absorbed. As band gap increases, compounds become coloured and eventually clear c.f. Diamond. If the band gap of a semiconductor is around 2-3 eV, light can excite electrons enough to cause conduction. This is used in solar cells and pigments. Transition Metal Oxides and Conduction WO3 and ReO3 are made up of ~M-O-M~ in all directions. The band structure is constructed by considering the overlap of the oxygen 2p orbitals and the metal 5d orbitals. σ bands and π bands are formed by overlap of orbitals. Below is the band structure for WO3 and ReO3. WO3 is a pale green semiconductor while ReO3 is a black metal. This is because of the extra electron from the d7 configuration of Re compared to the d6 configuration of W. Tungsten Bronzes Compounds with the formula NaxWO3. Made from reacting gaseous Na with solid WO3. Perovskite like structure with Na in 12 co-ordinate site. W-O-W structure is maintained therefore same band structure however the conduction band is now doped with electrons. Modifying Conductivity in Tungsten Bronzes Conductivity increases with the following modifications: Increase in x – Greater number of charge carriers Increase in temperature – as expected for a metal There is also a kink in conductivity at x = 0.75, this is due to a small change in the ordering of Na positions. ¼ cells has no Na+ this causes the unit cell to change slightly. The position of W is not affected but the change in structure results in an altered band structure. Transition Metal Oxides (Ti-Ni) These display NaCl type structures. Simplest band structure formed from overlap of d orbitals on adjacent metals. Due to octahedral coordination these bands are split into t2g and eg. This causes two sets of d bands. The band schematic on the left is also seen experimentally. However in each case the 3d band will be partially filled. However this is not the case for all metal oxides. As the nuclear charge increases the overlap between orbitals decreases. This causes narrow bands. The later transition metals (Mn to Ni) are known as MottHubbard Insulators. These have narrow bands which disfavour delocalisation and therefore the electrons are localised on the metal centres. This results in the energy required to move the conducting electrons being greater than the band with. This localisation of electrons can be proven by looking at the Optical Absorption Spectroscopy. The trace seen for hexa-aqua nickel is the same as seen for NiO crystals. Hence there is no real change between the localisation on the metal itself and when in the crystal form. Transition Metal Oxides vs Sulfides As shown by these graphics, TiO2 is an insulator while TiS2 acts as a metal. This is because 3p orbitals are much more diffuse and closer in energy to the 3d orbitals than 2p orbitals are. Hence there is overlap between orbitals and conduction can occur as there are vacant sites electrons can be put into with little energy needed for this promotion. Metal Oxides and Sulfides can be just as conducting as metals. Magnetism: Revision of Key Points Diamagnetism: A weak magnetic effect caused due to the rearrangement of electron spins to oppose a magnetic field, this is found in all substances. Paramagnetism: A magnetic effect caused by the presence of unpaired electrons in a material. Value will be close to 0 but there will be a net directionality. When Magnetisation, the magnetic response of a substance to a magnetic field, is plotted against applied magnetic field the following graph results. This shows how the spins of electrons within the compound align to the magnetic field when an external field is applied. The gradient of this graph is known as the susceptibility, χ. As temperature is decreased, thermal motion also decreases making the alignment of spins easier for a given applied magnetic field. Cooperative Magnetism Upon cooling an extended crystal structure, unpaired electrons can orient themselves spontaneously in the absence of a magnetic field. The spins of neighbouring atoms act in a cooperative manner. For this to occur, the temperature must be low enough for thermal motion to be overcome. The type of magnetism displayed by a compound can also be determined by the location of the x intercept when 1/χ is plotted against temperature. For a paramagnet this intercept value is 0, For a ferromagnet it is positive and is close to the Tc for that compound, For an antiferromagnet the x intercept is negative and is close to –TN. The Effect of Domains on Magnetism Within a crystal structure domains of magnetic fields can occur. Within each domain all of the spins ate aligned in the same direction however these domains are not aligned with each other until an external magnetic field is applied. Ferromagnetism in Transition Metal Dioxides In metal dioxides which take the rutile structure the formation of 3d bands of electrons occurs. Changing the transition metal changes the effect of these bands on the magnetism of the resulting crystal. CrO2 for example which has a half filled 3d band of electrons is ferromagnetic. This compound has a large density of states (wide bands of electrons). These electrons are delocalised and aligned to form a Pauli Paramagnet. MnO2, a Mott-Hubbard Insulator has narrow bands of localised electrons and is paramagnetic. Above TN metals behave as paramagnetic. Mn – Ni transition metal oxides have unpaired electrons, O2- is a weak field ligand. All are Mott-Hubbard insulators. Unpaired electrons are localised on metal centres M – O – M – O linkages allow transfer of magnetic information This diagram shows how antiferromagnetism can be induced throughout a metal crystal structure. As covalency of bonds increases the “super exchange” of spins becomes more effective. As Zeff increases, as does the ability to accept oxide electron density. As an example the T N of MnO is 122 K, whereas for NiO (a much greater Zeff) TN is 523K. Ferrimagnetism in Transition Metal Spinels Spinels are a ccp oxide lattice with 1/8th Td and ½ Oh sites filled by metal cations. A metal is Td, B metal is Oh. In Fe3O4 FeII and FeIII both sit on the octahedral site B. FeIII also sits on the tetrahedral site. This maximises the CFSE of the crystal structure. Ferrimagnetism is observed in this crystal. Antiparallel spins are observed however there is still a net magnetic moment. TfN = 858 K In the Oh B metal site, FeIII and FeII are ferromagnetically coupled via superexchange. More Examples of Ferrimagnetism in Transition Metal Oxide Spinels. ZnFe2O4: FeIII (3d5) CFSE = Zero (high spin) no preference ZnII (3d10) tetrahedral co-ordination preferred due to small size. Normal Spinel Ferromagnetic MgFe2O4: FeIII (3d5) CFSE = Zero (high spin) no preference MgII flexible but Oh preferred at 298 K. Inverse spinel: FeIII(Td)MgIIFeIII(Oh)O4 Ferrimagnetic TfN = 713 K Case Study 7 – Magnetic Strips Barium Ferrite – BaF2O4 Normal Spinel (Fe3+ high spin Oh) Ferromagnetic – One lattice of magnetic ions High coercivity: Difficult to switch magnetic moments therefore permanent storage of information.