here
advertisement
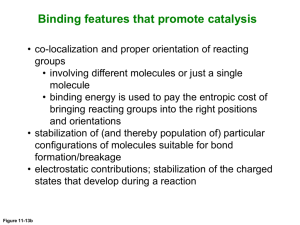
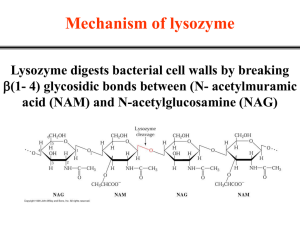
Molecular Organisation and Assembly in Cells By Sam Robson Micro-organisms have developed pathways to break benzenoid compounds. Bioremediation is cost effective, but long term effects are unknown. It is of great interest to understand the enzymatic pathways involved. Better understanding of metabolic pathways may lead to better usage of micro-organisms in pollution degradation. In the α,β-hydrolase family, a triad of amino acids (histodine, aspartate and serine) are conserved. They occur in the active site in close proximity to one another, where they interact to allow Serine to act as a nucleophile. This triad is present in MhpC, but it is unknown whether it has the same function as for the serine proteases. The serine can act as either a nucleophile or as a base. Serine as a nucleophile Previous results suggest the active site serine acts as a nucleophile. This would require the presence of an acyl intermediate step. Studies fail to show this intermediate, which suggests that the serine has some other function. Serine as a base Serine can also act as a base in hydrolytic attack on the carbon. This would require a gem-diol intermediate step. The exact mechanism is still unknown. 2,3-dihydroxyphenyl propionoic acid is one such compound. After the benzene ring is broken through oxidative meta-fission by MhpB, the enzyme MhpC hydrolytically cleaves the product. Hydrolytic cleavage of C-C bond is very rare. MhpC is a member of the α,βhydrolase family. A conserved catalytic triad is found in these proteins – see ‘The Catalytic Triad’. 25 Results suggest existence of ketointermediate step. Acts as electron sink. Insertion of hydrogen at C5 retains regiochemistry. Two possible mechanisms for attack at C6 – attack by water or active site nucleophile. Aim to study function by labelling with thiol directed fluorescent molecule (N(1-Pyrene) Maleimide), and observe fluorescence changes upon substrate binding. Structure of the thiol directed reagent N-(1-Pyrene Maleimide). Wildtype 5 0 190 Labelled 200 210 220 230 240 250 260 -5 Wavelength (nm) Used to check conformational changes upon labelling. Spectrum suggests predominately β-sheet formation. Shows 10 times less protein concentration than Bradford assay. Lack of proposed acyl enzyme intermediate suggests hydrolytic attack over nucleophilic attack. Proposed mechanism for C-C bond cleavage by MhpC. 3 2.5 Bradford assay relies on concentration of aromatic amino acids. Bradford reagent may therefore bind heavily to pyrene label. Apparent protein concentration would be much greater than actual concentration. The subsequent lack of protein in the fluorescence studies would give very low intensity readings. 2 LABELLED PROTEIN PROTEIN + SUBSTRATE 1.5 SUBSTRATE CONTROL BUFFER CONTROL 1 0.5 0 0 10 20 30 40 50 60 70 80 90 100 Time (secs) Graph shows change in fluorescence over time for MhpC labelled with N-(1-Pyrene) Maleimide. Time scale is too long to show any changes due to substrate binding. Initial dip when substrate present probably due to quenching of fluoresced light by the substrate. More accurate technique required to see fluorescence changes upon substrate binding. Structure of the amino acid residue cysteine. 10 -15 Intensity is very low - see ‘5: Circular Dichroism’. Oxidative meta-cleavage of benzene ring, followed by hydrolytic cleavage of product by MhpC. 15 -10 Both may involve active site serine – see ‘The Catalytic Triad’. Cysteine 270 found very close to triadic histodine in active site. Conserved in all α,β-hydrolase enzymes. Modification of Cys-270 by certain thiol directed reagents results in inactivation of protein. Function in enzymatic pathway is as yet unknown. 20 Ellipticity (millidegrees) C-C bonds in aromatic compounds are very hard to break. E. coli uses an aerobic degradative pathway for fission of aromatic compounds. Cleavage occurs between C5 and C6. Intensity Many pollutants contain aromatic carbon chains. Comparison of CD of wildtype and labelled proteins Suggest stopped-flow kinetics. Shows changes over a millisecond timescale. The addition of the fluorophore may have caused the Bradford assay to overshoot the protein concentration by a large amount. Thus the protein solutions used in the fluorescence studies were much more dilute than originally thought. This would explain the low intensities seen in absorbance and fluorescence studies. The labelling process seems to have worked, but the addition of the pyrene has affected the protein assay. If the experiment is to be repeated, the protein concentration must be ascertained using a method that will not be affected by the presence of the pyrene label. Acknowledgements and References Supervised by Prof. Timothy Bugg. Special thanks to Dr. Jian-Jun Li, Dr. Alison Rodger, Chen Li and Rachel Marrington. [1] Catalytic Mechanism of a C-C Hydrolase Enzyme: Evidence for a Gem-Diol Intermediate, Not an Acyl Enzyme, S.M. Fleming, T.A. Robertson, G.J. Langley and T.D.H. Bugg, Biochemistry 2000, 39, 1522-1531. [2] Purification, Characterization, and Stereochemical Analysis of a C-C Hydrolase: 2-Hydroxy-6-keto-nona-2,4-diene-1,9-dioic Acid 5,6Hydrolase, W.Y. Lam and T.D.H. Bugg, Biochemistry 1997, 36, 12242-12251.