Enthalpy of Formation & Combustion: Chemistry Lesson
advertisement

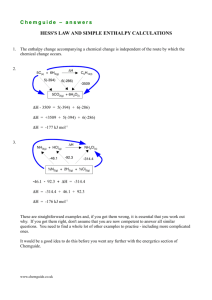
Title: Lesson 4 Enthalpy of Formation and Combustion Learning Objectives: – Calculate change in enthalpy of reactions using enthalpy of formation or combustion data How to construct an enthalpy change for the reaction Reactants - the elements that make up the product in their standard state. Theses need to be balanced (you can use fractions as the co-efficients) Product – The compound formed from the elements. Enthalpy value will be given in the IB booklet. Main Menu Main Menu Solutions Main Menu Main Menu Standard Enthalpy of Formation You need to know the ΔHθf for all the reactants and products that are compounds. The ΔHθf for elements is zero – the element is being formed from the element so there’s no change in enthalpy. Note: add when going ‘with’ an arrow, subtract when going against an arrow… (The questions will show arrows in a different directions within the cycle) http://www.yout ube.com/watch?v =c8Adft3M8mg Main Menu Using enthalpies of formation 8 of 36 © Boardworks Ltd 2009 Enthalpies of formation calculations 9 of 36 © Boardworks Ltd 2009 Energy of the system Calculate the enthalpy change of the combustion of ethanol Compound C2H5OH CO2 H2O ΔHf -277 -394 -286 C2H5OH + 3O2 2CO2 + 3H2O 2C + 31/2O2 + 3H2 Reaction path C2H5OH + 3O2 → 2CO2 + 3H2O Energy of the system Calculate the enthalpy change of the combustion of ethanol ΔHf -277 -394 -286 Compound C2H5OH CO2 H2O C2H5OH + 3O2 -277 2CO2 + 3H2O 2 x -394 2C + 31/2O2 + 3H2 Reaction path 3x -286 Energy of the system Calculate the enthalpy change of the combustion of ethanol ΔHf -277 -394 -286 Compound C2H5OH CO2 H2O C2H5OH + 3O2 -1369KJmol-1 -277 2CO2 + 3H2O 2 x -394 2C + 31/2O2 + 3H2 3x -286 Reaction path ΔHc = ∑ΔHofProducts - ∑ΔHofReactants = ( 2 x 394 + 3x -286 ) – ( -277 ) Energy of the system Calculate the enthalpy change of the reaction below CaO + H2O Ca(OH)2 What are the intermediates? Reaction path CaO + H2O → Ca(OH)2 Energy of the system Calculate the enthalpy change of the reaction below Compound CaO Ca(OH)2 H2O ΔHf -635 -986 -286 CaO + H2O Ca(OH)2 Ca(s) + O2(g) + H2(g) Reaction path CaO Now Ca(OH) ∆Hf 2 + Hwork 2O →out Energy of the system Calculate the enthalpy change of the reaction below ΔHf -635 -986 -286 Compound CaO Ca(OH)2 H2O CaO + H2O -65KJmol-1 -635 Ca(OH)2 -286 -986 Ca(s) + O2(g) + H2(g) This way is just following the direction of the arrows of the Hess cycle (elements towards reactants and products). Using the equation will give you the same answer! Reaction path -1 ∆Hf = - ((-635) CaO+ +(-286)) H2O → + (-986) Ca(OH) = -65KJmol 2 Calculate the enthalpy change of the reaction below Compound ΔH f CaO Ca(OH)2 H2O -65KJmol-1 Ca(OH)2 CaO + H2O -635 -286 -986 Ca(s) + O2(g) + H2(g) This canCaO be represented Ca(OH) a Hess2 cycle + H2O → as -635 -986 -286 Calculate the enthalpy change of the ΔH reaction below Compound CH NHNH +54.0 f 3 N2O4 CO2 H2O 2 -20.0 -393 -286 ∆H 4CH3NHNH2 + 5 N2O4 4CO2 + 12 H2O + 9N2 ∆H1 ∆H2 4C(s) + 9N2(g) + 12H2(g) + 10O2(g) ∆H = ∆H2 -∆H1 Calculate the enthalpy change of the ΔH reaction below Compound CH NHNH +54.0 f 3 2 N2O4 CO2 H2O -20.0 -393 -286 ∆H 4CO2 + 12 H2O + 9N2 4CH3NHNH2 + 5 N2O4 4 x 54 4 x -393 5 x -20 12 x -286 4C(s) + 9N2(g) + 12H2(g) + 10O2(g) ∆H1 = (5 x -20) + (4 x 54) = +116KJmol-1 ∆H2 = (12 x -286) + (4 x -393) = -5004KJmol-1 ∆H = ∆H2 -∆H1 ∆H = -5004 – (-116) CaO -1 2O → Ca(OH) 2 ∆H+=H-4888KJmol STANDARD ENTHALPY OF FORMATION REACTANTS SO2(g) + 2H2S(g) ΔHθr Route 1 PRODUCTS 3S(s) + 2H2O(l) ΔHθf(products) ΔHθf(reactants) 3S(s) + 2H2(g) + O2(g) ELEMENTS Step 1: Write the balanced equation for the reaction. This will be ΔHθr Step 2: Under the equation write a list of the elements present. This must be balanced STANDARD ENTHALPY OF FORMATION REACTANTS SO2(g) + 2H2S(g) ΔHθr Route 1 PRODUCTS 3S(s) + 2H2O(l) ΔHθf(products) ΔHθf(reactants) 3S(s) + 2H2(g) + O2(g) Step 3: ELEMENTS ΔHθf[SO2(g)] = -297 kJmol-1 ΔHθf[H2S(g)] = -20.2 kJmol-1 ΔHθf[H2O(l)] = -286kJmol-1 ΔHθf values give the enthalpy change going from the element to the compound Using Hess’ Law; Route 1 = Route 2 STANDARD ENTHALPY OF FORMATION REACTANTS SO2(g) + 2H2S(g) ΔHθr Route 1 ΔHθf(SO2) + 2 x ΔHθf(H2S) PRODUCTS 3S(s) + 2H2O(l) 3 x ΔHθf(S) + 2 x ΔHθf(H2O) 3S(s) + 2H2(g) + O2(g) Step 3: ELEMENTS ΔHθf[SO2(g)] = -297 kJmol-1 ΔHθf[H2S(g)] = -20.2 kJmol-1 ΔHθf[H2O(l)] = -286kJmol-1 ΔHθf values give the enthalpy change going from the element to the compound Using Hess’ Law; Route 1 = Route 2 STANDARD ENTHALPY OF FORMATION REACTANTS SO2(g) + 2H2S(g) ΔHθr Route 1 -297 + (2 x -20.2) PRODUCTS 3S(s) + 2H2O(l) (3 x 0) + (2 x -286) 3S(s) + 2H2(g) + O2(g) Step 3: ELEMENTS ΔHθf[SO2(g)] = -297 kJmol-1 ΔHθf[H2S(g)] = -20.2 kJmol-1 ΔHθf[H2O(l)] = -286kJmol-1 ΔHθf(s) is zero because its an element Using Hess’ Law; Route 1 = Route 2 STANDARD ENTHALPY OF FORMATION SO2(g) + 2H2S(g) ΔHθr Route 1 -297 + (2 x -20.2) Step 4: 3S(s) + 2H2O(l) (3 x 0) + (2 x -286) 3S(s) + 2H2(g) + O2(g) ΔHθf(SO2) + ΔHθf(H2S) + ΔHθr = 3ΔHθf(S) + 2ΔHθf(H20) -297 + (2 x -20.2) + ΔHθr = (3 x 0) + (2 x -286) ΔHθr = (3 x 0) + (2 x -286) – (-297 + (2 x 20.2)) = -234.6 kJmol-1 Using enthalpies of combustion 24 of 36 © Boardworks Ltd 2009 Enthalpies of combustion calculations 25 of 36 © Boardworks Ltd 2009 HESS’S LAW AND ENTHALPY OF FORMATION TASKS: 1. Complete the ‘Enthalpy of Formation’ worksheet 2. Complete the Hess’s Law cut and stick exercise Terry’s Cutting tip: Keep the left column and top row in one piece 1 One mole of carbon burns to give one mole of carbon dioxide, releasing 393.5 kJ. One mole of carbon burns to give one mole of carbon monoxide, releasing 110.5 kJ. Calculate the energy from burning one mole of carbon monoxide. Hf[CO2(g)] = -393.5 kJ mole-1 and Hf[CO(g)] = -110.5 kJ mole-1 CO(g) + ½O2(g) Hf[CO(g)] H CO2(g) Hf[CO2(g)] Alternative route = -110.5 = -393.5 Elements here because Hf given C(s) + O2(g) By Hess’s Law: What info do you have? Which equation to use? H = (SUM)H PRODUCTS - (SUM)H = (-393.5)-(-100.5) = - 283.0 kJ mole-1 REACTANTS 2 Use the Hf values given to calculate H of : CH3COCH3(l) + 4O2(g) 3CO2(g) + 3H2O(l) Hf[CO2(g)] = Hf[CH3COCH3(l)] = Hf[H2O(l)] = CH3COCH3(l) + 4O2(g) H - 394 kJmol-1 - 248 kJmol-1 - 286 kJmol-1 3CO2(g) + 3H2O(l) 3Hf[CO2(g)] + 3Hf[H2O(l)] Hf[CH3COCH3(l)] = -248 = 3(-394) + 3(-286) Elements here because Hf given 3C(s) + 3H2(g) + 4.5O2(g) H = (SUM)HPRODUCTS - (SUM)HREACTANTS = (3(-394) + 3(-286)) – (-248) = - 1792 kJ mole-1 What info do you have? Which equation to use? 3 Calculate Hf [CH4(g)], given Hf [CO2(g)] = -393.5 kJ mole-1 also = HC [C(s)] Hf [H2O(l)] = -285.8 kJ mole-1 also = HC [H2(g)] Hc[CH4(g)] = -890.3 kJ mole-1 C(s) + 2H2(g) CH4(g) H HC [C(s)] + 2HC [H2(g)] Hc[CH4(g)] = - 890.3 = (-393.5) + 2(-285.8) CO2(g) + 2H2O(l) = (SUM)HREACTANTS- (SUM)HPRODUCTS H = (-393.5 + 2(-285.8)) – (-890.3) What info do you have? Which equation to use? Oxides here because HC given = - 74.8 kJ mole-1 4 Calculate HR for : C2H4(g) + H2(g) C2H6(g), given Hc [C2H4(g)] = - 1410.8 kJ mole -1 = H c [H2(g)] - 285.8 kJ mole -1 H c [C2H6(g)] = - 1559.7 kJ mole -1 C2H4(g) + H2(g) + 3.5O2(g) C2H6(g) + 3.5O2(g) H Hc [C2H4(g)] + H c [H2(g)] H c [C2H6(g)] = -1559.7 = (-1410.8) + (-285.8) 2CO2(g) + 3H2O(l) H = (SUM)H REACTANTS- (SUM)H PRODUCTS = ((-1410.8) + (-285.8)) -(-1559.7) = - 136.9 kJ mole-1 Oxides here because HC given What info do you have? Which equation to use? NB H values calculated from bond energies are APPROXIMATE because: 1. 2. Average values used Gaseous state may not apply. Also, you may care to remember : Hr = Hf [PRODUCTS] - Hf [REACTANTS] Hr = HC [REACTANTS] - HC [PRODUCTS] Hr = E [REACTANTS] - E [PRODUCTS] Key Points ∆Hor is the enthalpy change of a reaction for molar quantities under standard conditions ∆Hor can be calculated from enthalpies of combustion/formation using Hess Cycles With formation, the arrows point up With combustion, the arrows point down Main Menu