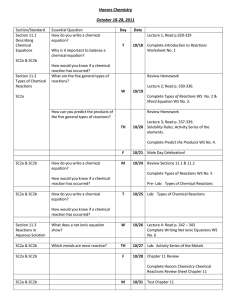
3.9.15.A.Tarvin.Week9.Unit5.LessonPlans

Monday
Content Standard:
SC2a Identify and balance the following types of chemical equations:
- synthesis
- decomposition
- single replacement
- double replacement
- combustion
SC2b Experimentally determine indicators of a chemical reaction, specifically precipitation, gas evolution, water production, and changes in energy to the system.
Essential Question:
How are the patterns in single replacement reaction, double replacement reaction, and combustion reaction used to predict products in the lab?
Musical Theme:
Ben Folds Five
Learning Activities:
[ 15 minutes] Writing, Balancing, Identifying Types of Equations Warm-Up [SC2a]
Summary: Students are invited to write and balance a chemical reaction as an individual to review the content learned on Friday and Monday. Teacher will facilitate a discussion of the correct process and answer. Students will have the opportunity to ask questions.
[ 30 minutes ] Tiered Assignment: Writing, Balancing, and Type of Chemical
Reactions [SC2a]
Summary: Teacher reviews assessment data related to writing chemical formulas, and students are assigned either the level one or level two assignment.
Level One: Students will primarily write equations, balance them, and identify the type of chemical reaction. Acceleration - They will be asked to attempt to predict the products of ONE chemical reaction.
Level Two: Students will begin by writing equations, balancing them, and identify the type of chemical reaction. Acceleration Focus – They will be asked to shift to predicting the products of THREE chemical reactions and write balanced equations to represent their predictions.
[ 30 minutes ] Inquiry Lab: Predicting and Recognizing Products (Standards SC2a and
SC2b)
Summary: Teacher will demonstrate the process of predicting products of a chemical reaction and illustrating the products at a particle level. Teacher will perform a chemical
reaction and model observation skills to recognize confirmation of predicted products.
Students will move to lab stations to carry out three chemical reactions. They’ll be asked to predict the products, illustrate the prediction at a particle level, carry out the reaction, recognize confirmation of their predictions, and illustrate products.
[ 15 minutes ] Formative Growth Check [SC2a]
Summary: Students will write balanced chemical equations for two chemical reactions.
Differentiation Plan:
Flexible grouping using diagnostic data: Yesterday’s exit question results and last night’s video quiz results informed the formation of today’s lab groups.
High expectations for all using critical thinking and the inquiry format
Assessment Plan:
Development of self-evaluation skills and monitoring own progress: Students will be invited to judge their own levels of understanding during our warm-up question.
Modified assessment for student needs: Diagnostic data is used to assign the flexible groups
Informal assessment of progress: Teacher will observe student mastery of today’s content standards by facilitating and observing the summarizing activity and marking the exit question [formative growth check].
Homework:
Watch Lesson 23 video, complete the notes, and take the post-video quiz.
Tuesday
Content Standard:
SC2a Identify and balance the following types of chemical equations:
- synthesis
- decomposition
- single replacement
- double replacement
- combustion
SC2b Experimentally determine indicators of a chemical reaction, specifically precipitation, gas evolution, water production, and changes in energy to the system.
Essential Question:
What patterns can I identify to predict what type of reaction is occurring? How can I analyze a reaction more closely to discover what atoms actually change and which stay the same?
Musical Selection:
The Middle
Learning Activities:
[ 15 minutes] Types of Chemical Reactions Quiz [SC2a]
Summary: Students will identify the 5 types of chemical reactions.
[ 20 minutes ] Tiered Inquiry Lab: Predicting and Recognizing Products (Standards
SC2a and SC2b)
Summary: Teacher will demonstrate the process of predicting products of a chemical reaction and illustrating the products at a particle level. Teacher will perform a chemical reaction and model observation skills to recognize confirmation of predicted products.
Students will move to lab stations to carry out three chemical reactions. They’ll be asked to predict the products, illustrate the prediction at a particle level, carry out the reaction, recognize confirmation of their predictions, and illustrate products.
Level One: Students will predict simple single and double replacement reactions. The actual reaction should confirm their predictions leaving little room for confusion.
Level Two: Students will encounter a reaction that does not match their predictions.
Teacher will enrich their understanding by explaining the quick decomposition of carbonic acid experienced in their reaction.
[ 30 minutes ] Predicting Products, Writing and Balancing Practice (Standards SC2a and SC2b)
Summary: Students will prepare for tomorrow’s quiz by continuing to predict products of combustion, single replacement, and double replacement reactions from their practice packets. They’ll write balanced chemical equations.
[ 25 minutes ] Writing Net Ionic Equations Outside of the Lab [SC2a extension]
Summary: Students will practice writing complete ionic and net ionic equations. They will also be asked to identify the spectator ions. [Net Ionic Equations handout]
Differentiation Plan:
Flexible grouping using diagnostic data: Yesterday’s exit question results and last night’s video quiz results informed the formation of today’s lab groups. Students scoring perfect or close were assigned to level two.
Appropriate challenge by differentiating product and accelerating content for
advanced students
High expectations for all using critical thinking and the inquiry format
Assessment Plan:
Common, Appropriate Formal Assessment (SC2a) Types of Chemical Reactions Quiz
developed with other HHS chemistry teachers for use in honors chemistry classes only.
Modified assessment for student needs: Diagnostic data is used to assign the level one or level two version of today’s activity to each individual student.
Informal assessment of progress: Teacher will observe student mastery of today’s content standards by marking the exit question [formative growth check].
Homework:
Prepare for tomorrow’s quiz for predicting products, writing, and balancing chemical reactions.
Review any videos.
Solve practice packet examples. [long worksheet 52, 56, 60, 61, 63]
Wednesday
Content Standard:
SC2a Identify and balance the following types of chemical equations:
- synthesis
- decomposition
- single replacement
- double replacement
- combustion
SC2b Experimentally determine indicators of a chemical reaction, specifically precipitation, gas evolution, water production, and changes in energy to the system.
Essential Question:
Have I mastered the skills and standards of the unit?
Musical Selection:
Relaxation Station
Learning Activities:
[ 40 minutes ] Predicting Products, Writing and Balancing Chemical Equations Quiz
[SC2a]
Summary: Students will be formally assessed on their abilities to predict products of
chemical reactions. They’ll then need to represent their predictions in balanced chemical equations.
[ 50 minutes ] Synthesizing the Standards Active Learning [SC2a and SC2b]
Summary: Students will visit three lab stations in homogeneous groups: 1. Indicators of a Reaction, 2. Identifying, Writing, and Balancing Equations, and 3. Net Ionic Equations.
Each station will invite students to experience the content in different ways: Actual - by carrying out a reaction, Verbal – write a description of the reaction, and Virtual – watching a video clip of controlling the rate of a reaction.
Differentiation Plan:
Modified assessment for student needs: Diagnostic data is used to assign the level one or level two version of today’s activity to each individual student.
Informal assessment of progress: Teacher will observe student mastery of content standards by observing students in the active learning stations.
Assessment Plan:
Common Formal Assessment: Teacher will use the quiz developed by HHS chemistry teachers. Grades will be recorded and papers returned.
Modified assessment for student needs: Diagnostic data is used to assign the level one or level two version of today’s activity to each individual student.
Homework:
Review any videos.
Solve practice packet examples.
Thursday
Content Standard:
SC2a Identify and balance the following types of chemical equations:
- synthesis
- decomposition
- single replacement
- double replacement
- combustion
SC2b Experimentally determine indicators of a chemical reaction, specifically precipitation, gas evolution, water production, and changes in energy to the system.
Essential Question:
Have I mastered the skills and standards of the unit?
Musical Selection:
None – Substitute Teacher
Learning Activities:
[ 30 minutes ]Complete the Synthesizing the Standards Lab [SC2a and SC2b]
Summary: Students will visit three lab stations in homogeneous groups: 1. Indicators of a Reaction, 2. Identifying, Writing, and Balancing Equations, and 3. Net Ionic Equations.
Each station will invite students to experience the content in different ways: Actual - by carrying out a reaction, Verbal – write a description of the reaction, and Virtual – watching a video clip of controlling the rate of a reaction.
[ 60 minutes ] Review of Unit Content [SC2a and SC2b]
Summary: Students will solve sample multiple choice and short answer test questions as a review of all unit content. The final 20 minutes of class will be used to go over answers to the review questions and assist with any misconceptions.
Differentiation Plan:
Flexible grouping: Students are continuing to work in their flexible groups to complete the lab and report.
Assessment Plan:
Development of self-evaluation skills and monitoring own progress: Students will be invited to judge their own levels of understanding during our warm-up question.
Homework:
Complete any review not completed in class and prepare for lab practical and test.
Friday
Content Standard:
SC2a Identify and balance the following types of chemical equations:
- synthesis
- decomposition
- single replacement
- double replacement
- combustion
SC2b Experimentally determine indicators of a chemical reaction, specifically precipitation, gas evolution, water production, and changes in energy to the system.
Essential Question:
What are the expected products of the chemical reaction? What indicators of a reaction are observed?
Musical Theme:
Ben Folds Five
Learning Activities:
[ 15 minutes] Cumulative Quiz
Summary: Students will demonstrate mastery of unit 4 skills by answering two short answer questions.
[ 75 minutes ] Lab Practical
Summary: Students will individually visit a lab station to predict products of two reactants, carry out a reaction, observe indicators, and determine if their predictions were confirmed by the reaction.
Differentiation Plan:
Flexible grouping using diagnostic data: Students will enter the lab practical according to their recent performances on quizzes and labs. Stronger students will be invited to go first, and others will have time to review prior to the practical.
Assessment Plan:
Common, Appropriate Formal Assessment (SC1b; SC3e; SC4a; SC4b): Lab practical and cumulative quiz developed with other HHS chemistry teachers for use in honors chemistry classes only.
Homework:
Review any videos.
Solve practice packet examples.