Transition Elements
advertisement

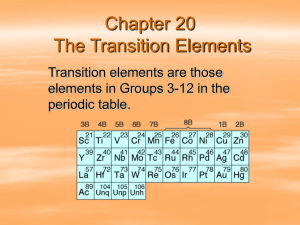
Transition Elements Chapter 5 Section 3 The Metals in the Middle Remember from Section 1 that the Transition elements are in groups 3-12 Transition elements don’t change properties as much as Representative elements do The Iron Triad Most transition elements are already combined with other transition metals— ores The iron triad is made of three elements in period 4. Iron- Fe, Cobalt- Co, and NickelNi Uses Transition Elements have a wide variety of uses: Tungsten- light bulbs Mercury- Thermometers Chromium- paint colors Ruthenium, Rhodium, palladium, osmium, iridium and platinum- catalyst • Catalyst- substance that makes a reaction happen faster but doesn’t change the reaction Inner Transition Elements WE have already sort of talked about the two series of inner elements---these are the funny ones on the bottom of the table The Lanthanide’s The Actinide’s Lanthanide Series Rare earth’s—because they were once thought to be scarce Soft metals Very hard to separate when they are in an ore together—they are so similar in properties Actinide Series Are ALL radioactive Thorium, Protactinium and uranium are the only NATURALLY occurring elements on Earth The rest are Synthetic elements Synthetic--- made in laboratories and nuclear reactors Dentistry Dentists have been using amalgam to fill cavities Amalgam is a mixture of silver, copper, tin and mercury • Since mercury is poisonous people have complained or stressed their concern Dentist now use a combination of chemically resistant resins, porcelains and composites. An after thought What are the two Inner transition series called and what is a trait of each?