R = b-OH (S)
advertisement
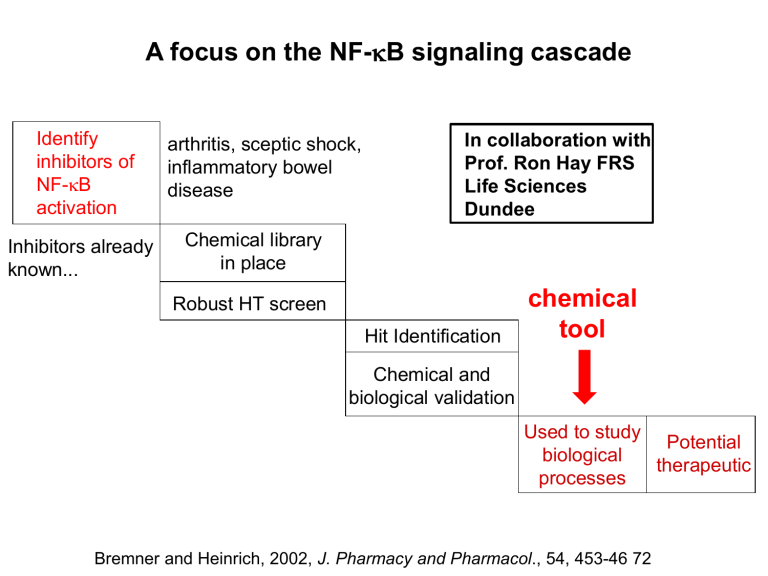
A focus on the NF-kB signaling cascade Identify inhibitors of NF-kB activation Inhibitors already known... arthritis, sceptic shock, inflammatory bowel disease In collaboration with Prof. Ron Hay FRS Life Sciences Dundee Chemical library in place Robust HT screen Hit Identification chemical tool Chemical and biological validation Used to study Potential biological therapeutic processes Bremner and Heinrich, 2002, J. Pharmacy and Pharmacol., 54, 453-46 72 A focus on the NF-kB signaling cascade Identify inhibitors of NF-kB activation Natural Product collection from Professor Alan Harvey, SIDR University of Strathclyde Chemical library in place Robust HT screen Hit Identification Chemical and biological validation NF-kB Luc Luciferase based Reporter gene assay Raquel Frade, Ron Hay Nick Westwood Used to study Potential biological therapeutic processes A focus on the NF-kB signaling cascade Identify inhibitors of NF-kB activation Natural Product collection from Professor Alan Harvey, SIDR University of Strathclyde Chemical library in place Tanacetum parthenium extract purification Raquel Frade Robust HT screen Hit Identification Chemical and biological validation Luciferase based reporter gene assay Raquel Frade, Prof Ron Hay FRS Nick Westwood structure assignment Dr Tomas Lebl Used to study Potential biological therapeutic processes A sesquiterpene lactone? ? solvent ? H H H O O H Assignment of the structure of the major component in the purified bioactive extract (1H) (13C) 1 - - 204.9 2 2.68 2.17 45.4 3 4.62 (4.65) - 71.8 (72.0) 4 - - 175.6 5 - - 138.0 6 4.94 - 77.2 7 3.11 - 43.4 8 1.91 1.77 28.4 9 2.46 - 40.8 10 - - 209.0 11 - - 140.9 12 - - 171.4 13 6.18 5.67 122.1 14 2.06 - 30.5 15 2.12 - 14.4 MS : 279 [M + H]+ Tomas Lebl Structurally related compounds O O R R1 O O R = b-OAc (S), R1 = H R = a-OAc (R), R1 = H R = b-OMe (S), R1 = H R = a-OMe (R), R1 = H R = b-OH (S), R1 = OH R = a-OH (S), R1 = OH R = b-OAc (S), R1 = OH R = a-OAc(S), R1 = OH Cl O O O O HO H Me O O O O R = b-OH (S), R1 = H iso-secotanapartholide From Artemisia rutifolia no report of a-OH isomer Zdero, Bohlmann et al., 1986, Phytochem., 25, 883-889; Jia et al., 1991, Phytochem., 30, 3033-3035; Oksuz, 1990, Phytochem., 29, 887-890; Toth et al., 2003, Tet., 59, 3729-3735. Structurally related compounds R = b-OAc (S), R1 = H R = a-OAc (R), R1 = H R = b-OMe (S), R1 = H R = a-OMe (R), R1 = H O O R R1 Cl O O O O HO R = b-OH (S), R1 = OH R = a-OH (S), R1 = OH R = b-OAc (S), R1 = OH R = a-OAc(S), R1 = OH O O H Me O O O O R = b-OH (S), R1 = H iso-secotanapartholide HO HO O O O OH H H H O O O HO O O O O O O secotanapartholides Knight et al., 1996, J.Chem.Soc., Perkin Trans. 1, 1979-1986 and references therein. A focus on the NF-kB signaling cascade Identify inhibitors of NF-kB activation Synthesis of authentic material to clarify stereochemistry issues at C3 Chemical library in place Robust HT screen Hit Identification 14 10 2 HO O O 1 9 3 8 4 5 6 7 15 11 O 13 Chemical and biological validation Used to study Potential biological therapeutic processes 12 O Edward Makiyi, Susan Cobb Proposed biosynthesis of the secotanapartholides OH OH O O O O HO H H O O O O O O and b-isomer and b-isomer OH O O H O O Bohlmann and Zdero, 1982, Phytochem., 21, 2543-2549; Knight et al., 1996, J.Chem.Soc., Perkin Trans. 1, 1979-1986; Rucker et al., 1992, Planta Med., 58, 293. Proposed biosynthesis of secotanapartholide OH O H O O O HO [BF3.OEt2] H O O O O Bohlmann and Zdero, 1982, Phytochem., 21, 2543-2549; Knight et al., 1996, J.Chem.Soc., Perkin Trans. 1, 1979-1986; Rucker et al., 1992, Planta Med., 58, 293. A biomimetic approach to iso-secotanapartholides? OH O O O H O O Artabsin Geissman and Winters, 1968, Tet. Lett., 27, 3145-3147. A biomimetic approach to iso-secotanapartholides? (1H) 1 - - 2 2.32, 2.69 2.32, 2.65 3 4.30 4.32 4 - - 5 - - 6 4.98 4.94 7 3.06 3.15 8 1.88, 1.96 1.87, 1.96 9 2.54, 2.61 2.52, 2.58 10 - - 11 - - 12 - - 13 5.67, 6.35 5.66, 6.35 14 2.15 2.15 15 2.15 2.13 OMe 3.41 3.41 O H O MeO OH O O O MeO O O O H O O Ji et al., 1991, Phytochem., 30, 583-587. An alternative semi-synthesis approach late stage oxidative cleavage with no protecting groups Synthesis of diastereomeric diols H OAc OH O conc. H2SO4 O H O cat. OsO4, NMO 97% H O O O-acetylisophotosantonic lactone Prof. Alex Slawin O THF, H2O 87% OH O H O O 3:1 inseparable mixture Buchi et al., 1966,J. Am. Chem. Soc., 88, 3403-3408. ... and then diastereomeric triols Prof. Alex Slawin ... and then diastereomeric triols Synthesis of 3b-OH-iso-secotanapartholide Greico et al., 1974, J.Org.Chem., 39, 119-120 Synthesis of epi-3a-iso-secotanapartholide .. and then for the comparison with the natural material Thanks to Professors Todorova, Marco, Lee, Ryu and Saliba Does anyone have any seco-tanapartholide? A comparison of iso-secotanapartholides with our isolated natural material 500MHz, CD3CN synthetic epi-3a-OH 3b-OH Natural source A focus on the NF-kB signaling cascade Identify key biological process Chemical library in place and 3b-isomer Robust HT screen Hit Identification Chemical and biological validation NF-kB Luc Used to study Potential biological therapeutic processes