Powerpoint Notes
advertisement

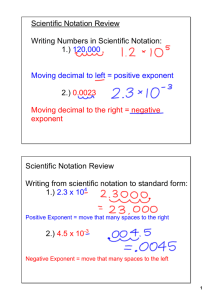
The scientific method consists of six interrelated processes: 1.Observation of a phenomenon 2. Develop a Question 3. Develop a Hypothesis (potential answer) 4. Experimentation 5. Data Analysis 6. Conclusions Worked out: Theory Further Experimentation Didn’t work out: New Hypothesis Develop New experimentation and theory 1. Observation Ex.- a “magic” box seemingly producing more water than possible DATA: the result of a measurement or observation Ex.- mass of a sample, time for a reaction to occur, temperature, length, volume QUALITATIVE DATA: general description (not exactly measured or can’t be measured) Ex.-color, odor, shape, texture, taste, smell; bigger/smaller; More?_____________________________ QUANTITATIVE DATA: answers “Exactly how much/little/fast/slow/big/heavy/hot/cold?” Ex.- pharmaceuticals (meds) development and dosage; feed ratios and amounts for your particular livestock; choke/brand/shell load for the most uniform pattern/density to take down whatever you are hunting; More?_______________________________ “Sheen” on water… “Strong Petroleum” odor “Stained” soils 2. Formulation of a question 3. HYPOTHESIS:a proposed reason/answer for what is observed that can be tested, an “educated guess” Observation of a phenomenon (something you see happening) calls for some explanation. The process of explaining observed behavior begins with a hypothesis. If the hypothesis survives extensive testing, it may attain the status of either a: THEORY: tested by more experiments and modified if necessary. A theory is considered successful if it can be used to make predictions that are true Ex.- Einstein’s theory of relativity or the atomic theory- a theory explains a broad principle of nature that has been supported over time. All theories are still subject to new experimental data and can be modified/changed. LAW: summarizes the results of many observations and experiments Ex.- Newton’s three laws of motion- If someone skydives from a plane, they will always wind up back at the Earth’s surface 4. EXPERIMENTATION:carefully designed testing process to reinforce (yes) or refute (no) the model system, the theory, or the hypothesis For the results of an experiment to be accepted, anyone must be able to consistently produce the same result by completing the experiment as directed INDEPENDENT VARIABLE: what you change in an experiment Independent variable should be the ONLY condition that affects the experiment’s outcome, everything else should be identical and controlled. Ex.- in an experiment to determine the amount of yeast that will create the fastest rise in dough, the amount of yeast would be the independent variable. DEPENDENT VARIABLE: changes in response to the independent variable Ex.-in an experiment to determine the amount of yeast that will create the fastest rise in dough, the height of the bread dough measured in the center would be the dependent variable. (It depends on the amount of yeast added). CONTROL OR CONTROL GROUP:the variable(s) in an experiment that must stay the same to eliminate false/misleading results Ex.- in an experiment to determine the amount of yeast that will create the fastest rise in dough, the flour type/amount, sugar type/amount, water temperature/amount, room temperature/pressure, surface on which the dough rises must all stay the same to be certain that the amount of yeast is causing the differences in height of dough. Also, during experiment set-up and completion, you MUST, MUST, MUST PREVENT CROSS-CONTAMINATION: indirect contamination caused by contact with a contaminated source Ex.- child with a severe peanut allergy that died from kissing her boyfriend who had eaten some peanut butter (or any food that doesn’t contain an allergen, like peanuts, that has been on equipment that processes peanuts and the nonpeanut food picks up peanut oils/dust, etc. that cause the non-peanut food to cause a person with a peanut allergy to react);equipment used for testing/sampling that isn’t decontaminated properly (water bailer and ungloved hands examples) In an experiment or test, if you do not prevent cross-contamination, YOUR RESULTS ARE NOT VALID (RELIABLE)!!!!! FYI It is an orange peel not a lemon!!!! 5. Data Analysis ALWAYS THINK about your results, especially in lab situations. Do they make sense? If not, figure out why. Retest. Rethink your controls/variables. What else might be affecting your results (crosscontamination/another source)??? Ex.-Analytical results show contamination where repeated testing has shown no contamination has ever existed on a property. (email string Mrs. Ashcraft showed us about a groundwater well that was being reported as contaminated even though 10+ years of data showed it had never been) 6. CONCLUSION: a judgment based on the information obtained in an experiment MODEL: a drawing or 3-D representation of a hypothesis, theory or law Many hypothesis, theories and laws are expressed with mathematical equations that may confuse all but the best of mathematicians (anyone watch the show “Numbers”??). Models simplify complex ideas and provide a visual image to help us process information. Measurements are quantitative information. They are more than just numbers, however. For example, a cook could not communicate a recipe by saying “Add 1 salt, 2 sugar and 3 flour.” Measurements report specific quantities represented by common units. Nearly every measurement is a number plus a unit. UNIT: a unit of measurement compares what is to be measured with a previously defined STANDARDIZED size. A unit defines the basic quantity of mass, volume, time or other quantity being measured. Ex.- foot, inch, meter, pound, gram. A “foot length” was used centuries ago to mark off distances, but this system didn’t work as the foot lengths were all different because everyone’s feet were different. So, an agreement was made on the standard “foot” length and measuring implement’s (rulers) were developed. Scientists all over the world have agreed on a single measurement system called The System of International Units, abbreviated SI. This system was adopted in 1960 by the General Conference on Weights and Measures. More than 1 SI PREFIXES:are prefixes added to base units to represent quantities that are smaller or larger than the base unit ACCURACY: refers to the closeness of a set of measurements to the actual/correct value of the quantity measured PRECISION: the degree of agreement between multiple measurements (though they may not be the correct value) PERCENTAGE ERROR: comparing the accuracy of an individual value or average experimental value quantitatively with the correct/accepted value Percentage error= Value experimental-Value accepted x 100 Valueaccepted Ex.- The actual density of a certain material is 7.44 g/cm3. A student measures the density of the same material as 7.30 g/cm3. The percent error is found as follows: Percentage error= 7.30 g/cm3-7.44g/cm3 x 100 = -1.9% 7.44g/cm3 UNCERTAINTY: the degree of doubt in a single measurement Uncertainty is where SIGNIFICANT FIGURES, or the number of meaningful digits, is applied. The number of meaningful digits is determined by the measuring device being used. SCIENTIFIC NOTATION: also referred to as EXPONENTIAL NOTATION. It involves the representation of a number as a power of ten. Numbers are written in the form M x 10n, where the factor M is a number greater than or equal to one (and the correct amount of significant digits) but less than 10 and n is a whole number. Ex.-2,300 can be written as 2.3 x 103 or 2.3 E103 (To move the decimal in the scientific notation back to the original number you would have to move it three places to the right, so the exponent is “3”. Implied positive signifies you are moving to the right or more positive.) 0.0023 can be written as 2.3x10-3 or 2.3 E10-3(To move the decimal in the scientific notation back to the original number you would have to move it three places to the left, so the exponent is “-3” Negative signifies you are moving to the left or more negative.) Rule: to convert a number to scientific notation, the original decimal point is moved to the left or right so that the final number includes one digit to the left of the decimal followed by however many digits to the right necessary to achieve the correct amount of significant digits followed by the exponent signifying the number equal to the number of places from which the original decimal was moved. (see examples) Mathematical Operations using Scientific Notation 1. Addition and Subtraction: Values (n factor/exponent) must be the same. If they are not, adjustments must be made to the values so that all exponents are equal. Once exponents are equal, the M factors can be added or subtracted. Ex.- 4.2x104 kg + 7.9x103 kg 1. Adjust to make exponents equal, so 4.2 x104kg + .79x104kg 4.99x104 kg 2. Then just add, keeping exponent as is 3. Check significant digits. This should only have 2, as your original measurements only had two, so round to two significant digits. FINAL ANSWER= 5.0x104kg 2. Multiplication: The M factors are multiplied and the exponents are ADDED algebraically (following integer rules for addition). Ex.- (5.23x106mm)(7.1x10-2mm)= 1. multiply 5.23x7.1= 37.133 2. adjust to correct significant digits, so 37 3. add exponents 6+(-2), so 4 4. put it all together, so 37x10437x104mm2(don’t forget, when multiplying, the unit will also be squared or cubed) 5. record in correct scientific notation format, so FINAL ANSWER is 3.7x105mm2 3. Division: The M factors are divided and the exponent of the denominator is SUBTRACTED algebraically (following integer rules for subtraction)from the exponent of the numerator. Ex.- (5.23x106mm)/(7.1x10-2mm)= 1. 5.23/7.1= .73661972 2. adjust to correct significant digits, so .74 3. subtractdenominator exponent from numerator exponent 6-(-2) [remember, when subtracting integers, you ADD it’s opposite], so it is actually 6+2=8 4. put it all together, so .74x108 5. record in correct scientific notation format, so FINAL ANSWER is 7.4x107 CHEMISTRY:The study of the composition, structure and properties of matter and the changes it undergoes including the energy changes that accompany those changes. CHEMICAL: any substance that has a definite composition Therefore, ALL matter—living and nonliving, natural or artificial —has a chemical basis. A common misconception exists that any “chemical” is artificial or unnatural. MATTER: anything that has mass and occupies space. ENERGY: The ability to do work to accomplish some change. There are three main states of Matter: SOLID: consists of particles that are close together and have a regular and predictable pattern of particle arrangement (crystalline). A solid has both fixed volume and fixed shape. Solids have very strong attractive forces and are fixed in position compared to liquids and gases. LIQUID: consists of particles closer together than in a gas and has a definite volume but no definite shape—it takes the shape of its container. Liquid molecules can flow around each other. GAS: consists of particles that are widely separated and has no definite shape—it will expand to fill any container. Note- Gases are still considered “fluids”, as they have the ability to flow. An important fourth state of matter is known as PLASMA: a high temperature physical state of matter in which atoms lose most of their electrons, particles that make up atoms. Ex.- Plasma is found in fluorescent light bulbs and stars All matter can have chemical and physical properties: CHEMICAL PROPERTY: relates to a substance’s ability to undergo changes that TRANSFORM it into DIFFERENT substances Ex.- The ability of a substance (wood/charcoal, etc.) to burn PHYSICAL PROPERTY: can be observed or measured without changing the identity of the substance Ex.- melting point, boiling point (not the same as catching something on fire), freezing point A CHANGE OF STATE is a physical change of a substance from one state to another. Change of State Process Name Example Solid--Liquid Melting Ice--Water Solid-- Gas Sublimation Dry Ice—CO2 Gas Liquid-- Solid Freezing Water--Ice Liquid--Gas Vaporization Gas--Liquid Condensation Gas--Solid Deposition Water—Water Vapor Water Vapor-Water Water Vapor--Ice DENSITY: a characteristic physical property of a substance that is the ratio of mass to volume Density= mass or D=m volume V Objects/substances with more density will sink in objects/substances with less density. MASS: a measure of the amount of matter (physical property). Mass is measured using a balance (scale). NOTE: Mass and weight are not the same. Mass is determined by comparing the mass of an object with a set of standard masses that are part of the balance and does not change. Whereas, …. WEIGHT: is a measure of the gravitational pull on matter. Weight will change depending on the force of gravity on an object. VOLUME: the amount of space occupied by an object CHEMICAL CHANGE/REACTION: one or more substances are converted into different substances Ex.- rusting iron (iron combines with oxygen in the air); metal corrosion because of acid rain PHYSICAL CHANGE: a change in a substance that does not involve a change in the identity of the substance Ex.- grinding, cutting, melting and boiling something (a material) The boundaries between physical and chemical changes aren’t always clear. For example, while most chemists would consider the dissolving of sucrose (a sugar) into water to be a physical change, many chemists would consider dissolving table salt in water as a chemical change. ATOM: the smallest unit of an element that maintains the chemical identity of that element. ELEMENT: pure substance that cannot be broken down into simpler, stable substances and is made of one type of atom. It can have one or more atoms of the same kind. Ex.- Carbon. Carbon is an element containing one kind of atom. All discovered elements are represented on the periodic table. COMPOUND: a substance that can be broken down into simple stable substances. Each compound is made of the atoms of two or more elements that are chemically bonded. Ex.- Water. Water is made of two elements, hydrogen and oxygen. MIXTURE: is a blend of two or more kinds of matter, each of which retains its own identity and properties (physical change) Ex.- air that we breathe is a mixture of gases and particulate matter, sweet tea, wood, blood HETEROGENEOUS MIXTURE: a mixture that is not uniform throughout (“hetero” = “different”—remember heterozygous traits from Biology [Rr]?) Ex.- a tossed salad, no bake cookies, blood HOMOGENEOUS MIXTURE: a mixture that is uniform throughout, also known as SOLUTIONS(“homo” = “same”--remember homozygous traits from Biology [rr]?) Ex.-sweet tea, chocolate milk